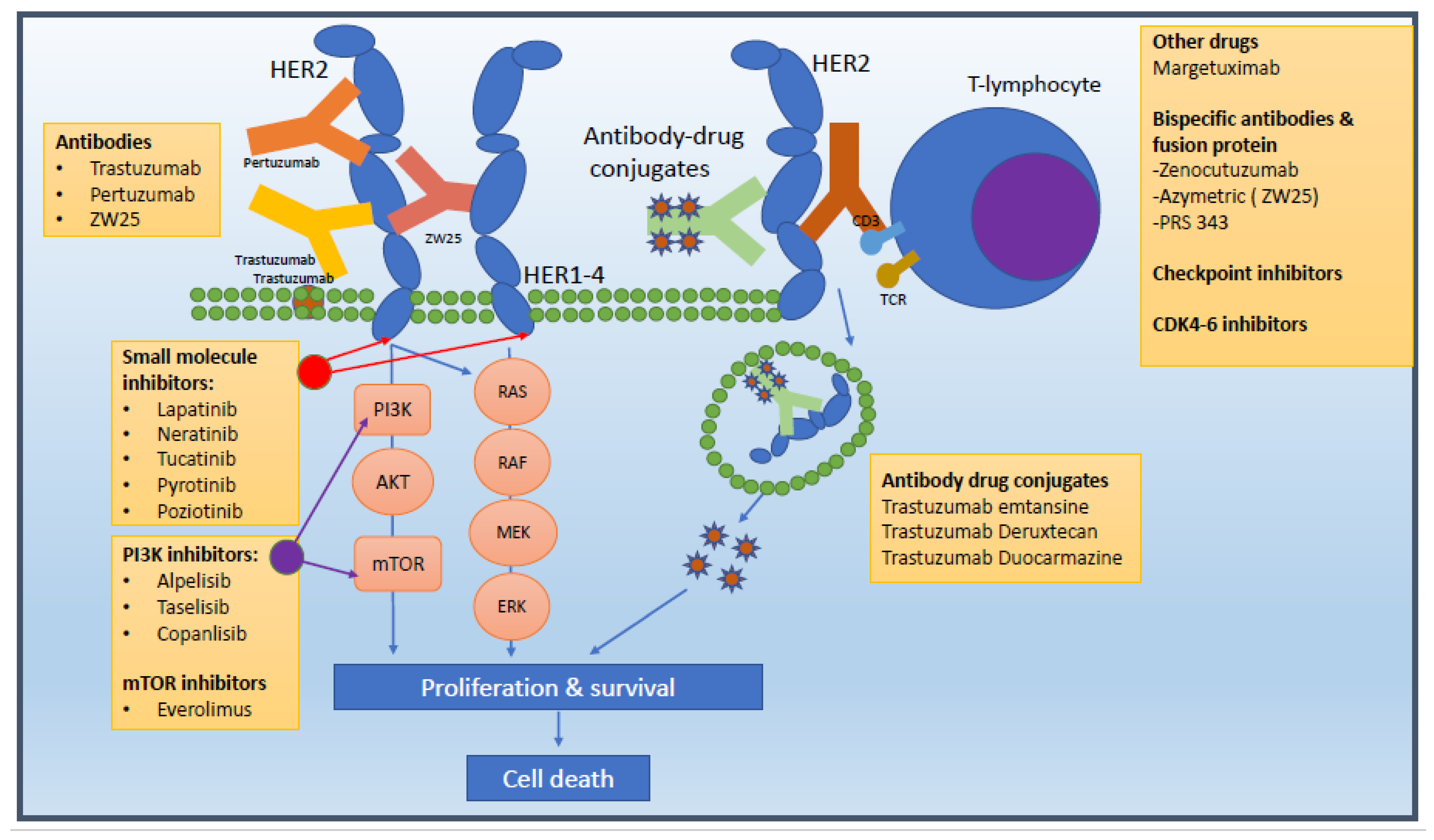
Trastuzumab Deruxtecan Plus Pertuzumab: A New Benchmark in HER2-Positive Breast Cancer Treatment
The recent presentation at the 2025 American Society of Clinical Oncology annual meeting has sparked conversation among oncologists and healthcare professionals. The data emerging from the DESTINY-Breast09 trial redefines the first-line standard of care for HER2-positive metastatic breast cancer. In this opinion editorial, I aim to examine the study’s findings, compare them to prior treatment regimens, and offer insights into the potential real-world impact of this combination therapy.
For more than a decade, the THP regimen—comprising a taxane with trastuzumab and pertuzumab—has been the mainstay of treatment. However, with a median progression-free survival (PFS) of 18.5 months, clinicians have long wished for an option that could extend patients’ lives significantly. The innovative use of trastuzumab deruxtecan, an antibody-drug conjugate, in combination with pertuzumab appears to offer exactly that. The study demonstrated a remarkable 44% reduction in the risk of disease progression or death over two years, suggesting that the combination is not only statistically significant but also clinically transformative.
Understanding the Study Design and Really Getting Into the Data
When we take a closer look at the trial design of DESTINY-Breast09, it becomes clear that the research team left no stone unturned in addressing the tricky parts of clinical endpoints. The trial randomized 1,157 patients, comparing the new combination against the standard THP regimen. The primary endpoint was progression-free survival, assessed by blinded independent central review, with secondary endpoints including overall survival, objective response rate, duration of response, and safety.
The trial results were compelling. At a median follow-up of 29 months, patients receiving the trastuzumab deruxtecan and pertuzumab combination experienced median PFS by blinded review of 40.7 months versus 26.9 months for those on THP. Investigator assessments echoed these encouraging trends, with consistent benefits seen across various patient subgroups. This consistency strongly supports the idea that the new combination may soon become the key standard of care for metastatic HER2-positive breast cancer.
Long-Term Outcomes in HER2-Positive Metastatic Breast Cancer
One of the most attractive aspects of this new therapy is its potential for durable, long-term outcomes. The median duration of response was longer with the novel combination, clocking in at 39.2 months compared to 26.4 months in the THP group. When we look at the table below, the stark differences in clinical outcomes become even more pronounced:
| Outcome | Trastuzumab Deruxtecan + Pertuzumab | THP Regimen |
|---|---|---|
| Median PFS (months) | 40.7 | 26.9 |
| Risk Reduction (PFS) | 44% lower risk | – |
| Duration of Response (months) | 39.2 | 26.4 |
This table encapsulates the fine points of the benefits we see with the new combination. The improvement in progression-free survival is not a minor tweak—it represents a significant leap, providing patients with more time and a better quality of life.
Weighing the Safety Profile and Side Effect Considerations
No new cancer treatment can be lauded without considering the safety profile. Both the combination treatment and the standard THP regimen showed similar rates of grade 3 or higher treatment-emergent adverse events (around 63% and 62%, respectively). However, there is one concerning twist: the occurrence of adjudicated drug-related interstitial lung disease or pneumonitis. The new combination had a significantly higher incidence at 12.1% compared to just 1% in the THP group.
This disparity calls for cautious optimism. While most cases of lung disease were not severe, the possibility of interstitial lung disease remains an off-putting challenge. Clinicians must work through the delicate bits of patient monitoring and risk assessment, ensuring that those who are most likely to experience severe side effects are carefully managed. This aspect of care is both critical and nerve-racking, emphasizing the need for careful patient selection and ongoing surveillance.
Addressing the Tricky Parts of Treatment Sequencing and Patient Selection
Even as we celebrate the potential of this breakthrough, important questions remain regarding treatment sequencing and patient selection. Clinicians are now left to figure a path through the tangled issues associated with timing and duration of therapy. The study indicated that many patients who benefitted from the new combination had de novo metastatic disease or had hormone receptor–positive disease. However, the trial also revealed some variations in previous treatment histories—some patients had not received what would be considered contemporary HER2-directed therapy before enrollment.
These differences signal that the real-world application might require additional fine-tuning. Experts like Claudine Isaacs have pointed out that while the data looks promising, we need to sort out whether all patients should receive this new treatment as a first-line option. Some questions to consider include:
- Should the new combination be offered to everyone with metastatic HER2-positive breast cancer?
- Can we adopt a more selective approach based on individual medical histories and biomarker profiles?
- How should therapy duration be optimized across different patient groups?
Addressing these subtle twists will be essential in ensuring that the promise seen in clinical trials translates into everyday practice.
Patient-Centered Considerations: Personalizing the Therapeutic Journey
The shift towards a regimen involving trastuzumab deruxtecan and pertuzumab is not just a scientific breakthrough—it holds profound implications for patients’ lives. For many facing a diagnosis of metastatic HER2-positive breast cancer, the expansion of progression-free survival translates directly to more time with their loved ones and the opportunity to enjoy life’s everyday moments. This is a key benefit that resonates well beyond clinical numbers.
When discussing the new treatment approach, it is important to get into how patients view and manage the nerve-racking aspects of their therapy. Personalizing the therapeutic journey means that healthcare providers must communicate clearly about both the promising outcomes and the potential risks. This two-sided conversation allows patients to feel fully informed and empowered—a crucial aspect for those standing at the threshold of treatment decisions.
Reflections on the Historical Evolution of HER2-Positive Breast Cancer Treatment
Looking back at the evolution of treatment strategies for HER2-positive breast cancer, it is impossible to ignore the monumental shifts that have occurred over the decades. The landmark CLEOPATRA study, which established THP as the standard, was a major step forward. Yet, even that regimen has its twists and turns—its PFS metrics, though durable, leave room for improvement, particularly when compared with what the new combination offers.
The introduction of trastuzumab deruxtecan has reshaped the treatment landscape by providing an option that not only promises a longer PFS but also a sustained duration of response. It is a reminder of how ongoing research and innovation continue to steer through the tangled issues of cancer care, moving us ever closer to personalized, high-impact treatment solutions. This historical context is important when discussing advancements, as it shows how far we have come and where the future may lead.
Impact on Clinical Practice and the Healthcare Ecosystem
With new data emerging, healthcare professionals are now left to get around the practical challenges of incorporating such therapies into daily oncology practice. The emerging evidence suggests that incorporating trastuzumab deruxtecan plus pertuzumab will inevitably lead to changes in treatment protocols. This shift is more than just a change in drug regimens—it signals a broader transformation in how metastatic HER2-positive breast cancer is managed.
Healthcare providers must collaborate to figure a path that minimizes the nerve-racking issues related to toxicity management and treatment sequencing. An effective strategy will involve multidisciplinary collaboration among oncologists, pulmonologists, pharmacists, and nurse practitioners. By taking a closer look at patient therapy logs and long-term outcomes, the medical community can gradually refine best practices to ensure that every patient benefits from the latest breakthroughs.
Economic Aspects and the Promise of Reduced Financial Toxicity
While the clinical benefits of the new regimen are clear from a progression and survival standpoint, another dimension to consider is the potential economic impact of this treatment innovation. Oncology biosimilars have already shown promise in reducing the financial burden of cancer care. With the introduction of trastuzumab deruxtecan combined with pertuzumab, there is an opportunity to reduce long-term healthcare costs by extending the time patients spend without disease progression.
The economic aspect is full of problems for many patients who face overwhelming treatment costs alongside their medical challenges. By potentially reducing the frequency of hospitalizations and prolonging treatment intervals, this new combination therapy might ease some of the hidden complexities that make managing metastatic breast cancer so economically taxing. In this context, long-term financial relief becomes as much a part of the conversation as the side effect profile and PFS improvements.
Real-World Considerations: From Clinical Trials to Everyday Practice
Transitioning treatments from the controlled environment of clinical trials to the more unpredictable realm of real-world practice is always an intimidating challenge, loaded with issues that extend beyond statistical benefits. Many factors could influence how well the findings from DESTINY-Breast09 translate in diverse healthcare settings. Some of these include:
- The variation in prior treatment protocols among patients.
- The presence of comorbid conditions which may complicate therapy.
- Access to appropriate monitoring and management of side effects, particularly for interstitial lung disease.
- Differences in insurance coverage and patient affordability for new treatments.
Working through these complicated pieces means that multidisciplinary teams must stay alert to the little details that affect patient outcomes. Initiating a robust post-marketing surveillance program may be necessary to monitor how these therapies perform in a broader patient population. Such efforts can help clinicians refine their approach to ensure that the promising trial results are not lost in translation when applied in everyday practice.
Strategies for Managing Adverse Effects and Enhancing Patient Safety
Given the potential for serious side effects like interstitial lung disease, it becomes super important for clinicians to have a proactive approach in managing and mitigating treatment-related toxicities. Safety remains a critical aspect when considering any new oncologic regimen. The approach may include:
- Regular imaging and pulmonary function tests to detect early signs of lung toxicity.
- Patient education sessions that inform individuals about the signs and symptoms of potential adverse events.
- Implementing clear standards for dose adjustments or temporary treatment holds in response to emerging side effects.
This multi-pronged strategy can help steer through the nerve-racking issues associated with side effects while ensuring that patients continue to have access to the benefit of prolonged progression-free periods. Moreover, having detailed treatment protocols in place can ease the confidence of both providers and patients, making the path to recovery slightly less intimidating.
Reflections on the Role of Biomarkers and Future Response Markers
Another fine area of exploration going forward is the identification of biomarkers that can better predict which patients are most likely to benefit from the addition of trastuzumab deruxtecan. As modern medicine continues to rely on precision and personalized care, the development of response markers is super important for optimizing treatment sequencing.
Future research should poke around the possibility of integrating biomarker testing into the decision-making process for metastatic HER2-positive breast cancer. Such tests could help in:
- Identifying patients with a higher risk of developing interstitial lung disease.
- Determining who might achieve longer progression-free survival with the combination therapy.
- Customizing the duration and intensity of treatment regimens for individual patients.
These refined markers could also help clinicians when it comes to quickly steering through the subtle details of treatment response, ensuring that everyone gets the most benefit while minimizing exposure to overly aggressive therapy.
Exploring the Fine Points of Real-World Data Versus Clinical Trials
While clinical trials are fundamental in establishing a treatment’s efficacy and safety, there is a growing emphasis on understanding how treatments perform in real-world settings. The controlled environment of a trial typically does not capture the many little twists that occur in everyday clinical practice. In real-life scenarios, patients may have coexisting conditions, variations in previous treatments, and diverse genetic profiles that all interact with the treatment protocol.
This gap between clinical trials and real-world data is full of problems that require diligent research. By collecting observational data and analyzing outcomes from community oncology centers, clinicians can get a better picture of how to optimize the combination of trastuzumab deruxtecan and pertuzumab. Understanding these subtle distinctions is key to ensuring that the promising benefits observed in the trial translate into improved survival and quality of life for patients outside of research settings.
Getting Into the Economic Impact: A Closer Look at Treatment Costs
The economic considerations of adopting a new treatment regimen are not to be overlooked. The costs of advanced therapies often place a heavy burden on patients, particularly when insurance coverage is limited or when traveling for specialized care is required. The introduction of trastuzumab deruxtecan alongside pertuzumab raises important questions regarding cost-effectiveness and long-term financial sustainability.
Some of the key aspects to consider include:
- The direct cost of the new drugs versus the standard THP regimen.
- Potential savings from extended progression-free survival, which might reduce hospitalizations and emergency care visits.
- The broader economic benefits from improved quality of life and the ability to maintain daily activities longer.
While upfront costs may appear intimidating, the potential for overall cost savings in healthcare, coupled with improved patient outcomes, provides a compelling case for the integration of this new combination into standard practice.
Lessons Learned and the Road Ahead in Cancer Care Innovation
The journey of treating metastatic HER2-positive breast cancer has been marked by several important milestones—and each new development brings with it both promise and challenging questions. As we take a closer look at the data and the fine details of the new combination therapy, it becomes clear that the next few years will be filled with both excitement and opportunities to fine-tune treatment protocols.
There are several lessons that the oncology community can embrace from the DESTINY-Breast09 trial:
- High-impact therapies emerge from continuous innovation and rigorous research.
- The transition from clinical trials to real-world practice requires thoughtful consideration of individual patient factors.
- Comprehensive patient monitoring and proactive adverse event management are essential for optimizing outcomes.
- Economic and quality-of-life benefits are as critical in evaluating new treatments as traditional clinical endpoints.
These insights underscore the need for a dynamic and flexible approach in cancer care. Researchers, clinicians, and policymakers must all work together to figure out the best ways to implement these findings, ensuring that every patient has access to optimal therapy with manageable side effects.
Understanding the Patient Perspective: Trusting in Innovation Amid Uncertainty
For patients, the news about this new combination therapy offers hope. It is easy to get lost in the tricky parts of clinical outcome data and detailed trial designs, but at its core, the conversation is about people—real individuals facing a life-altering diagnosis. When a treatment promises to prolong the time a patient lives without disease progression, it brings with it a renewed sense of possibility.
However, the journey for many patients also involves coping with the overwhelming reality of side effects and treatment uncertainty. This experience is full of problems and can sometimes feel intimidating, especially when novel drugs come with their own sets of unique risks, such as lung toxicity. Empowering patients through education and clear communication is therefore a must-have element of successful treatment strategies. With a proactive and patient-centered approach, healthcare providers can help patients navigate the twists and turns of their therapeutic options while maintaining trust in the innovation that modern oncology has to offer.
Charting a Course Through the Tangles of Treatment Decisions
The decision to implement a new therapy in a clinical setting is rarely straightforward. Healthcare providers must weigh the benefits of a longer progression-free survival and durable response against the potential for side effects and the complicated bits of managing treatment sequencing. This decision-making process is akin to finding your way through a maze that features both empirical data and the unpredictable patterns of real-world practice.
In practice, this means fostering dialogue among the entire healthcare team—oncologists, nurse practitioners, pharmacists, and even patient advocates. Such collaboration is key to sorting out the overlapped issues and ensuring that every patient receives personalized treatment. By pooling expertise and learning from both clinical studies and everyday experiences, practitioners can fine-tune how best to integrate trastuzumab deruxtecan plus pertuzumab into treatment pathways, all while remaining sensitive to the unique needs and challenges of individual patients.
Integrating Innovations into a Broader Patient-Centered Oncology Framework
Emerging therapies should be viewed not in isolation but as vital pieces of an integrated treatment paradigm. The combination of trastuzumab deruxtecan and pertuzumab fits into an evolving framework that values precision medicine, patient engagement, and multidisciplinary care. The data supports its efficacy, yet the real progress lies in how the oncology community weaves new breakthroughs into the everyday reality of patient care.
This approach involves holistic practices such as:
- Regular multidisciplinary meetings to evaluate patient cases.
- Tailoring treatment plans to individual patient profiles and biomarkers.
- Ensuring that discussions about economic impact, quality-of-life improvements, and supportive care are integrated into treatment decisions.
By moving away from a one-size-fits-all mentality and embracing a more nuanced, patient-centered model, the healthcare system can better tackle those complicated pieces of treatment decisions. In doing so, we not only improve clinical outcomes but also enhance overall patient satisfaction and trust in modern oncologic care.
Conclusion: A New Era in HER2-Positive Breast Cancer Care?
As we wrap up this discussion, it is clear that the introduction of trastuzumab deruxtecan plus pertuzumab in the first-line setting heralds a promising new era for HER2-positive metastatic breast cancer patients. The trial data, which indicates longer progression-free survival and a more durable response, represents a significant clinical milestone. While some nerve-racking issues remain—such as optimizing treatment sequencing and managing side effects—the overarching message is one of cautious optimism.
Each advancement in cancer care is a piece of a larger puzzle, one that requires dedicated effort from all stakeholders to figure a path through the tangled issues. It is up to clinicians, researchers, and patient advocates to work together in taking the wheel toward a future where treatment is not only more effective but also more personalized and sustainable. The progress we see today builds on decades of hard work and innovation, and it reassures us that even in the face of overwhelming challenges, transformative breakthroughs are possible.
In summary, the new combination therapy paves the way for a future where metastatic HER2-positive breast cancer may be managed more effectively, with fewer compromises and greater patient benefit. The fine points discussed here—from treatment design and long-term outcomes to real-world application and economic impact—underscore the importance of staying flexible, informed, and compassionate in our approach to cancer care. As we continue to dig into the emerging data and learn from ongoing research, there is every reason to believe that the future will bring even more refined treatment strategies that are tailor-made for each patient’s journey.
Ultimately, trusting in innovation while keeping an eye on the practical, real-world challenges is the key to navigating this exciting yet complicated landscape. With every new study, every refined protocol, and every patient success story, we move one step closer to a world where a diagnosis of metastatic breast cancer no longer heralds a death sentence but instead signals the beginning of a fight backed by evidence, empathy, and cutting-edge science.
Originally Post From https://www.ajmc.com/view/trastuzumab-deruxtecan-plus-pertuzumab-improves-pfs-vs-standard-care-in-her2-breast-cancer
Read more about this topic at
ENHERTU® Granted Breakthrough Therapy Designation …
FDA Grants T-DXd Breakthrough Therapy Designation in …