Revolutionizing Cancer Prediction with Digital Cell Simulations
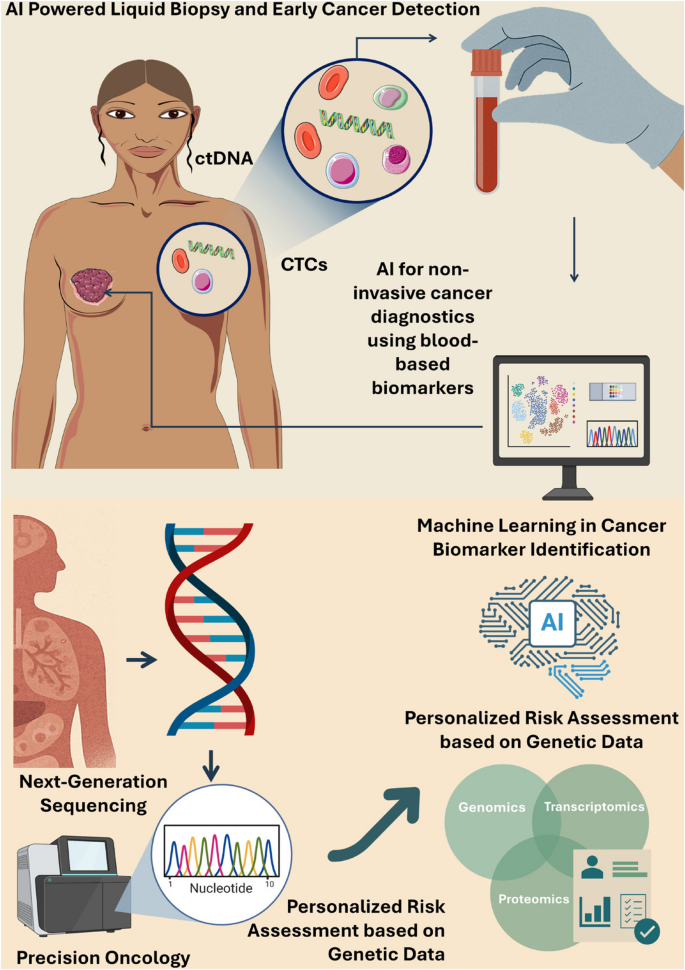
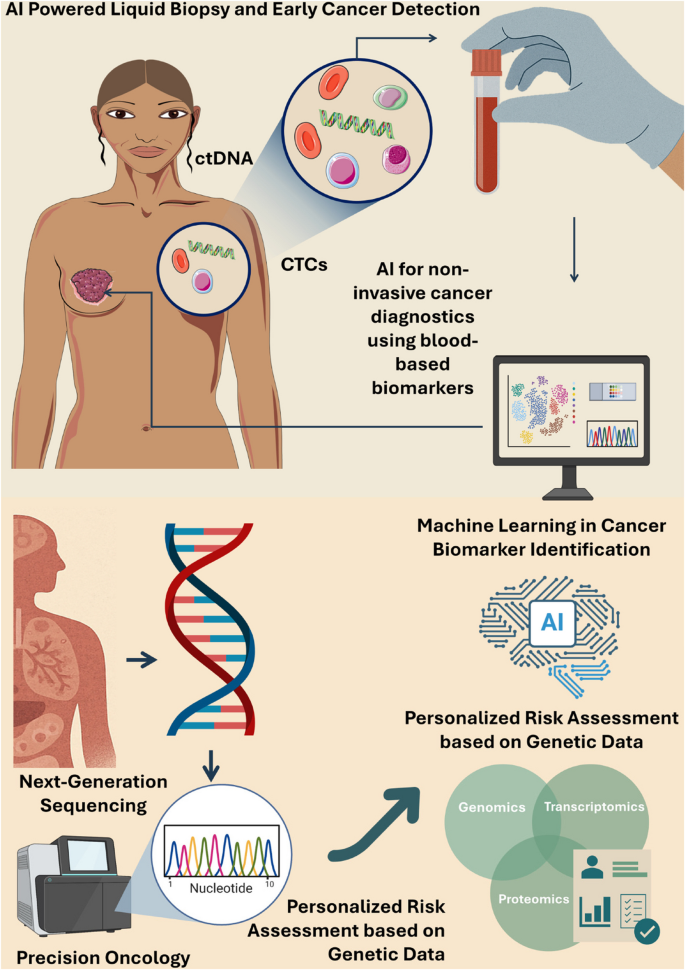
The world of cancer research is witnessing a transformation reminiscent of the shift from analog to digital weather forecasting. Today, scientists are combining advanced genomics with innovative computational models to predict how tumors might evolve over time. This breakthrough approach has the potential to reshape how we diagnose, treat, and manage cancer, offering a personalized lens into each patient’s condition.
In this editorial, we take a closer look at a pioneering method developed by researchers at the University of Maryland School of Medicine. This method, which uses a digital “forecast” to simulate cell behavior, provides a window into the intricate interplay between cancer cells and their environments. Throughout our discussion, we will dig into the origins of this innovation, examine its significant benefits, consider the tricky parts of the research, and discuss its implications for personalized medicine.
Harnessing the Power of Genomics and Artificial Intelligence
At the heart of this breakthrough is the integration of patient genomics with an easy-to-understand “hypothesis grammar.” By using computational models akin to weather prediction systems, scientists can now simulate the small distinctions and communication patterns that occur among cells. Such simulations provide a real-time digital laboratory where researchers can test hypotheses, experiment with therapeutic strategies, and predict cancer progression in a manner that is as clear as forecasting an approaching storm.
Key Ingredients in the Digital Cell Laboratory
This innovative research rests on a few super important elements that work together efficiently:
- Patient Genomics: Detailed genomic data that captures the individual differences among patients, essential for teasing out the tangled issues inherent in cancer’s development.
- Plain-Language “Hypothesis Grammar”: A unique language tool that translates biological behaviors into computational commands, making it easier for scientists to define and follow the subtle parts of cell communication.
- Computational Modeling: Software that acts like a digital twin of a patient’s tissue, capable of simulating how cells interact, evolve, and sometimes misbehave, much like predicting the path of a storm.
- Integration of Clinical Data: Incorporation of experimental and clinical results from bench teams ensures that the simulations aren’t merely theoretical, but hold practical insights for patient care.
In essence, these components allow researchers to easily figure a path through the overwhelming array of data, making it possible to explore how cancer might behave under various scenarios.
Simulating Tumor Growth: A New Frontier
One of the most fascinating aspects of this research is the concept of simulating tumor growth over time. Traditional biomedical studies often provide just a snapshot—a single picture of the cell’s state—at one moment. In contrast, digital simulations offer a dynamic view, much like a time-lapse movie that reveals the progressive evolution of cellular communities.
How Digital Forecasting Works in Cancer Research
The approach draws parallels with meteorology: just as weather models predict storms by simulating the atmosphere, these computational tools predict how tumors develop by mimicking cell-to-cell interactions. Let’s look at some of the fine parts of this process in a clear and organized manner:
| Step | Description |
|---|---|
| Data Collection | Gathering patient-specific genomic data along with tissue samples to serve as the foundational input for the models. |
| Hypothesis Grammar Application | Using plain-language rules to translate biological observations into computer-readable instructions. |
| Simulation Setup | Establishing a virtual cell environment where algorithms simulate cell growth, signaling, and interactions over time. |
| Outcome Prediction | Running the simulation to predict how cancer cells might grow and respond to various therapies. |
| Data Validation | Cross-checking simulation results with clinical outcomes and laboratory data to refine predictive accuracy. |
This table highlights the methodical approach researchers are taking to peel back the tangled issues of cancer, one step at a time.
Personalized Medicine: Creating a Digital Twin of the Patient
One of the super important benefits of this research is the potential to create a “digital twin” for each patient. A digital twin is essentially a precise virtual replica of a patient’s tumor and surrounding tissues, allowing researchers to experiment with treatment strategies before they are applied in the clinical setting.
Benefits of a Digital Twin in Cancer Treatment
The idea of a digital twin could transform the way oncologists approach cancer therapy. Here are some key advantages:
- Tailored Treatments: By simulating how a patient’s cancer might evolve or respond to a specific drug, physicians can select therapies that are most likely to work for that individual.
- Risk Reduction: Trying treatments in a virtual environment reduces the risk of adverse reactions since potential side effects are spotted ahead of time.
- Cost Efficiency: Virtual simulations can help avoid the nerve-racking, expensive trial-and-error phase in clinical treatments, thereby saving time and resources.
- Enhanced Understanding: Creating a digital twin allows scientists and doctors to get into the nitty-gritty of how cancer develops and how the immune system reacts—knowledge that can be extended to other diseases as well.
In this way, the digital twin approach is not just a technical marvel; it marks a fundamental shift in how we understand and manage disease, offering a more personalized road map for patient care.
Illuminating the Dark Corners of Cancer with Hypothesis Grammar
Central to this breakthrough is a concept known as “hypothesis grammar.” Developed through interdisciplinary collaboration, this tool translates the data from genomics into plain, everyday language that computers can process. This removes some of the intimidating barriers between medical jargon and software algorithms, making it easier for scientists from different fields to work together.
Why Plain-Language Hypothesis Grammar Matters
The use of everyday language in describing cellular behavior offers several unique advantages:
- Interdisciplinary Communication: Scientists, clinicians, and engineers can all work on the same page, avoiding misunderstandings that often arise from overly technical language.
- Accessible and Transparent: By reducing the use of arcane phrases, the system demystifies the biology behind the simulation, making the method accessible to a broader community of researchers.
- Flexible Integration: This approach is designed to be modular, meaning that it can be adapted easily to model other diseases and biological processes beyond cancer.
- Speed and Scalability: Simple language rules expedite the process of setting up simulations, allowing researchers to bring new hypotheses to the testing phase more quickly.
By translating the subtle details of cell communication into simple instructions, hypothesis grammar allows for a more inclusive and collaborative research environment, where diverse experts can contribute their little twists and insights towards a common goal.
Challenges and Tricky Parts in Computational Oncology
Despite its promise, this innovative approach is not without its tricky parts. The process of modeling cancer digitally involves several intricate steps that are loaded with potential issues. From the overwhelming amounts of genomic data to the nerve-racking complexity of cell signaling networks, scientists face many challenges along the way.
Overcoming Data Overload and Cellular Communication Hurdles
Addressing these challenges involves both technical prowess and creative problem-solving. Some of the most significant hurdles include:
- Sheer Data Volume: Genomic data is vast, and processing this information can be like finding your way through a labyrinth filled with confusing bits and twists and turns.
- Complex Cell Interactions: The way cancer cells talk to each other and to normal cells involves a network of signals that can be overwhelming to model accurately.
- Integration of Varied Data Types: Combining spatial transcriptomics, protein expression data, and clinical observations is a nerve-racking task that requires robust algorithms and high computational power.
- Validation of Models: Ensuring that digital predictions match real-world outcomes requires careful alignment with clinical data, which is itself often full of problems and delicate nuances.
To overcome these challenges, researchers have adopted a multi-pronged strategy that includes cross-disciplinary collaboration, iterative testing, and the continuous refinement of their computational models. The process is akin to carefully untangling a complex ball of yarn—each thread must be examined, understood, and then reassembled to form a coherent picture of the disease process.
Bridging the Gap: From Bench Science to the Clinic
The translational aspect of this research is perhaps one of its most promising features. By merging the worlds of bench science and clinical practice, these computational models are paving the way for a new era in precision oncology. The journey from a controlled laboratory environment to practical, life-changing treatment for patients is a challenging one, but the potential rewards are enormous.
Steps to Integrate Virtual Simulations into Patient Care
For these digital simulations to truly transform cancer treatment, they must be seamlessly integrated into existing clinical workflows. Here are some of the pivotal steps needed to achieve this integration:
- Clinical Validation: Extensive validation studies are needed to ensure that simulation predictions accurately reflect patient outcomes. This involves parallel testing with traditional diagnostic methods.
- Data Standardization: Establishing uniform protocols for genomic data collection and processing is essential. Uniform data allows simulations to be both reliable and reproducible across different institutions.
- User-Friendly Interfaces: Developing interfaces that clinicians can easily use is key to translating complex simulations into actionable treatment plans. These tools need to simplify the complex pieces of cell interaction without obscuring critical details.
- Collaborative Research: Continuous collaboration between computational scientists, laboratory researchers, and medical professionals ensures that the model remains both accurate and relevant. This teamwork provides the framework for smoothing out the subtle differences between digital predictions and real-world behavior.
These strategies, when implemented together, can create a robust pipeline from initial data collection to practical clinical application. The digital twin concept, in particular, stands to revolutionize how doctors approach cancer therapy, moving from a one-size-fits-all model to one that is highly individualized.
Implications for Future Research and Technology Adoption
The implications of this work extend far beyond just cancer. The same tools and principles have the potential to shed light on numerous other diseases, including autoimmune disorders, neurodegenerative conditions, and even metabolic syndromes. With digital simulations becoming more refined, the possibility of virtually testing emerging therapies before actual clinical trials becomes increasingly real.
Expanding the Horizons: From Cancer to Other Diseases
Here are a few ways in which the digital simulation approach could influence a wider spectrum of medical research:
- Immune System Modeling: Just as the simulation captures the subtle details of cancer cell communication, it can also be used to forecast immune system behavior. This could be a game changer in understanding conditions like rheumatoid arthritis or multiple sclerosis.
- Neuroscience Applications: Researchers at Johns Hopkins have already taken initial steps by applying similar methods to simulate brain development. Such models could eventually help us predict the onset of neurological disorders with greater precision.
- Precision Drug Testing: Beyond oncology, virtual simulations can streamline the drug discovery process for many diseases. Testing drugs on a digital twin can uncover potential effects and side effects early in the development cycle.
- Complex Disease Interactions: Many diseases are not isolated events; they involve interactions between multiple body systems. Advanced simulations can help disentangle these tricky parts, allowing researchers to see the cascading effects of an intervention.
By extending these models to other branches of medicine, researchers can build a comprehensive digital laboratory that addresses a wide range of biological systems. This approach not only enhances our understanding of individual diseases but also helps bridge the gaps between different fields of medical science.
Reflecting on the Journey: A Collaborative Triumph
The progress made in simulating cellular behavior is a testament to the power of collaborative, interdisciplinary research. By bringing together experts from computational science, genomics, clinical medicine, and even weather prediction, the team at the University of Maryland has charted a new course in the field of precision oncology.
The Collaborative Spirit Behind the Innovation
This breakthrough is the result of a multi-year, multi-lab project that underscores the value of teamwork and shared expertise. A few aspects that stand out include:
- Shared Knowledge: Combining insights from fields as diverse as weather prediction and immunology has led to the creation of a model that is both innovative and practical.
- Cross-Disciplinary Dialogues: By fostering conversations between computational experts and bench scientists, researchers have managed to break down traditional barriers, paving the way for a more integrated approach to medical research.
- Open Source Commitment: The decision to make the hypothesis grammar open source ensures that scientists worldwide can access and build upon this work, further expanding its impact.
- Clinical Partnerships: Collaborations with institutions such as Johns Hopkins University and Oregon Health Sciences University help validate the models, ensuring that they remain grounded in clinical reality.
This spirit of collaboration not only accelerates the pace of discovery but also distributes the burden of working through the nerve-racking challenges inherent in pioneering research. It is an excellent reminder that modern medicine is as much about community and shared effort as it is about technological prowess.
Addressing the Overwhelming Data Challenges in Genomic Research
Genomic research produces an enormous amount of data, and harnessing this information presents its own set of intimidating challenges. The integration of these vast datasets into something that can be meaningfully simulated requires both advanced algorithms and a careful approach to data management.
Tackling the Data Deluge: Strategies and Solutions
Some of the most critical strategies to manage and make sense of overwhelming genomic data include:
- Data Standardization Protocols: Establishing streamlined processes for data collection can help ensure consistency across large data sets, easing the burden of integration.
- Efficient Computational Methods: Leveraging high-performance computing and advanced machine learning algorithms can help researchers get around the tricky parts of data overload.
- Collaborative Data Sharing: Open-source platforms and shared databases allow the global research community to contribute and validate findings, smoothing out many of the little details associated with data variance.
- Iterative Refinement: As more data is collected and integrated, continuous refinement of models ensures they remain accurate and clinically relevant.
By addressing these data challenges head-on, researchers can make significant strides in turning raw genomic data into actionable clinical insights that have the potential to guide real-world treatment decisions.
Looking Ahead: The Future of Computational Models in Healthcare
As we stand at the intersection of genomics and computational modeling, the possibilities for future research are both exciting and expansive. The approach of using digital forecasts in oncology paves the way for embracing similar techniques across healthcare disciplines. With each step forward, we get closer to a future where precision medicine is the norm rather than the exception.
The Road to Broader Clinical Adoption
For computational models to become a staple in patient care, several super important milestones must be achieved:
- Enhanced Model Accuracy: Ongoing research must focus on refining simulations so that the predictions align even more precisely with clinical outcomes.
- Integrative Platforms: Developing comprehensive platforms that integrate diverse data types—from proteomics to patient histories—will help bridge the gap between laboratory research and bedside applications.
- User-Centered Design: Clinicians need tools that are intuitive and practical. Creating user-friendly interfaces that distill complex simulated data into clear, actionable insights is crucial.
- Regulatory Advancements: As digital simulations begin to influence treatment decisions, establishing clear regulatory frameworks will be key to ensuring that these technologies are used safely and effectively.
As these models continue to evolve, it is essential to maintain strong dialogue among researchers, clinicians, and regulatory bodies. This ensures that the insights gleaned from digital simulations translate seamlessly into preventive measures, tailored therapies, and improved patient outcomes.
Embracing a New Era in Precision Oncology
The journey from traditional laboratory snapshots to dynamic, digital forecasts represents a profound shift in how we understand and treat cancer. With the promise of simulating tumor behavior and creating digital twins, the approach is not just a technical achievement but a leap toward personalized, patient-centric care.
Why Digital Simulations Are a Game Changer in Cancer Research
Digital simulations offer a fresh perspective on the battle against cancer, with several key takeaways driving home their importance:
- Improved Predictive Accuracy: By modeling the growth and evolution of cancer cells, researchers can anticipate how tumors might respond to treatment, reducing the guesswork involved in therapy selection.
- Reduced Clinical Risks: Simulated trials minimize the need for risky, early-stage human testing, offering a safer route to discovering effective treatment regimens.
- Resource Optimization: Virtual testing environments allow for rapid experimentation, significantly cutting down the costs and time associated with traditional clinical trials.
- Personalized Therapy Plans: Digital twins enable the customization of treatment, ensuring that each patient receives a regimen tailored to their unique genetic makeup and tumor characteristics.
These strengths underscore the transformative potential of digital simulations in not only oncology but across an array of medical fields. It is a step toward ensuring that medicine remains as agile and responsive as the diseases it seeks to combat.
Conclusion: Charting a New Course in Healthcare Innovation
As we look toward the future, the integration of genomics and computational modeling heralds a new era of precision and personalization in medicine. The innovative approach developed by the team at the University of Maryland School of Medicine is a prime example of how merging digital simulations with clinical data can open up new avenues for addressing some of the most overwhelming issues in cancer treatment.
By leveraging a blend of detailed genomic data, plain-language hypothesis grammar, and robust computational models, researchers are not only demystifying the tangled conversational patterns between cells but also paving the way for more effective, individualized treatment strategies.
This digital forecasting method, though not without its tricky parts and overwhelming challenges, represents a significant stride in our understanding of cancer. It exemplifies the power of collaborative, cross-disciplinary research and the importance of integrating clinical insights with high-powered computational tools. In doing so, we stand to revolutionize patient care, moving ever closer to the goal of truly personalized medicine.
Ultimately, as more data is gathered and techniques are refined, the potential applications extend beyond oncology. The ability to simulate complex biological systems opens up opportunities in neurology, immunology, and beyond—ushering in a future where many diseases can be better understood, predicted, and managed through digital innovation.
As we continue to work through and figure a path from disruption to deep transformation in healthcare, the journey of digital cell simulations promises not just new scientific insights, but also a more hopeful outlook for patients worldwide. In a landscape often loaded with challenges, this research stands as a beacon of progress—a demonstration that with the right tools, even the most tangled issues of cell behavior and cancer growth can be unraveled.
The road ahead is undoubtedly filled with both opportunities and nerve-racking challenges. Yet, with every simulation that more accurately mirrors real-life patient data, we take another step toward a future where the predictive power of artificial intelligence in healthcare is not only a scientific marvel but a practical, life-saving tool. Through sustained collaboration, innovative thinking, and a steadfast commitment to patient-centered research, this digital revolution in cancer diagnosis and treatment can—and will—transform modern medicine for generations to come.
Originally Post From https://www.sciencedaily.com/releases/2025/07/250726234433.htm
Read more about this topic at
Predictive Modeling: The Basics – CBIIT
Personalization of cancer treatment using predictive …