The Promise and Challenges of Melatonin in Stem Cell Therapy
Melatonin, a naturally produced hormone, has intrigued scientists for years. Its potential to influence the behavior of stem cells—especially in relation to the formation of new blood vessels—is a subject filled with both promise and tricky parts. In an era where cardiovascular diseases remain one of the leading causes of death globally, finding new ways to enhance tissue regeneration is essential. In this editorial, we take a closer look at what melatonin brings to the table in the field of regenerative medicine, particularly its impact on angiogenesis in stem cells, while also discussing some of the tangled issues and nerve-racking challenges associated with its use.
Many researchers and clinicians are on the lookout for strategies that improve the survival rate and functional integration of stem cells when they are deployed in harsh ischemic conditions, such as those found during myocardial infarction. Because melatonin is known for its antioxidant, anti-inflammatory, and even tumoricidal properties, it has emerged as a super important agent in modulating stem cell therapy. The hormone’s pleiotropic effects can support tissue regeneration by improving blood flow via angiogenesis.
Understanding the Fine Details of Melatonin’s Role in Angiogenesis
When we talk about angiogenesis in regenerative medicine, it refers to the formation of new blood vessels from existing ones. This process is an essential component of tissue repair following injuries and ischemic events. Stem cells, whether they be mesenchymal stem cells (MSCs) or endothelial progenitor cells (EPCs), have a natural ability to initiate this process, either by directly differentiating into functional vascular cells or by releasing pro-angiogenic factors in a paracrine manner.
Melatonin’s role in these processes is subtle yet multi-layered. On one hand, it helps protect stem cells from the overwhelming and often intimidating environment of oxidative stress—a major issue in ischemic tissues. On the other hand, melatonin tweaks the secretome of these cells, boosting the release of growth factors like vascular endothelial growth factor (VEGF), hepatocyte growth factor (HGF), and basic fibroblast growth factor (bFGF). This not only accelerates the formation of blood vessels but also supports the survival and integration of the transplanted stem cells.
Several studies indicate that incubation of stem cells with melatonin results in enhanced expression of anti-oxidative enzymes such as catalase and superoxide dismutase. These enzymes are key in scavenging free radicals and mitigating the damaging effects of reactive oxygen species (ROS), which are common in post-ischemic tissues.
How Melatonin Protects Stem Cells in Stressful Environments
One of the primary hurdles in stem cell therapy is the limited survival of these cells after transplantation into regions afflicted by inadequate blood supply. The harsh conditions can promote cell death through apoptosis and necrosis. Melatonin steps in as an effective guard by scavenging ROS and engaging cellular pathways that prevent apoptotic changes.
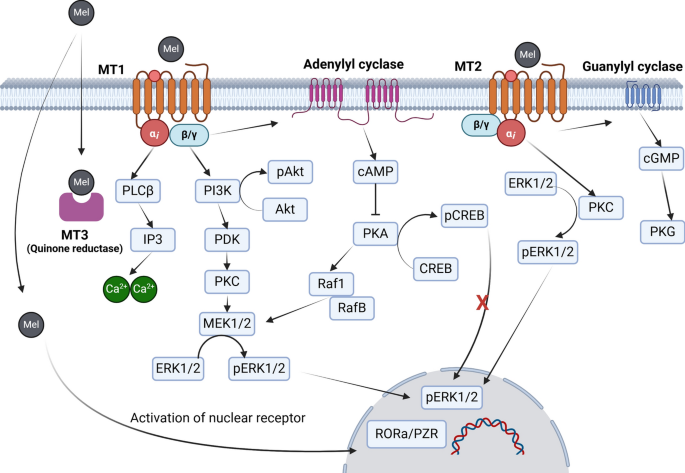
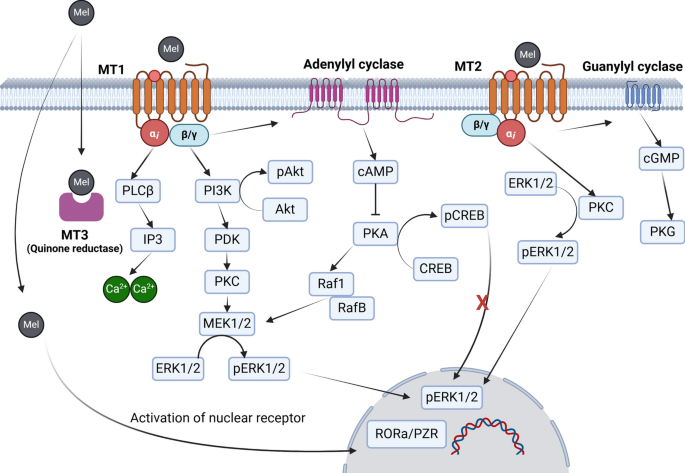
For example, in experimental models, melatonin-treated mesenchymal stem cells exhibit increased survival rates compared to their untreated counterparts. This improved cell viability is partly due to the activation of membrane-bound melatonin receptors (MT1 and MT2), which trigger downstream signaling cascades such as the PI3K/Akt pathway. This pathway is key to cell survival and proliferation, and its activation encourages the cells to persist even in a hostile microenvironment.
Furthermore, melatonin not only bridges the gap in stem cell survival but also boosts their ability to produce pro-angiogenic factors. A higher level of VEGF production, for instance, allows for better blood vessel formation around the injured tissues, ultimately leading to improved blood perfusion and tissue recovery. Researchers have even observed that when stem cells are pretreated with melatonin, tissue fibrosis and necrosis in ischemic models are significantly reduced.
Melatonin and Its Dual-Action: Promoting and Inhibiting Angiogenesis
It is fascinating to note that melatonin’s effect on angiogenesis is not purely linear. Depending on the dose and context, melatonin can either stimulate or inhibit the angiogenic process. At physiological levels, this hormone tends to enhance angiogenesis by enabling stem cells to secrete key growth factors and protect themselves from oxidative damage. However, at much higher, pharmacological dosages, melatonin might actually slow down the process by interfering with certain signaling pathways.
This dual-action quality makes melatonin a particularly interesting yet challenging adjuvant in stem cell therapies. Its effects could be described as dose-dependent. For instance, while a moderate amount of melatonin boosts EPC mobilization and microvascular recovery in diabetic limb ischemia models, too high a concentration might dampen the pro-angiogenic behavior of these cells. This delicate balance requires researchers and clinicians to carefully consider dosing and timing when incorporating melatonin into treatment protocols.
The phenomenon can be summarized in the table below:
| Melatonin Dosage | Effect on Angiogenesis | Stem Cell Response |
|---|---|---|
| Physiological Levels | Enhances angiogenesis | Improved viability, increased VEGF secretion, protection from oxidative stress |
| High/Pharmacological Levels | May inhibit angiogenesis | Possible downregulation of migratory and differentiation properties |
The Role of Mitochondria and Sirtuins in Stem Cells Under Melatonin Influence
Melatonin is also well-known for its relationship with mitochondria—the powerhouses of the cell. In stem cells, proper mitochondrial function is paramount to ensure energy production, especially under stressful conditions. Melatonin is not only produced by mitochondria but also helps regulate their activity. Improved mitochondrial function means enhanced regulation of reactive oxygen species and better energy supplies to sustain rapid cell growth and differentiation.
Moreover, melatonin’s impact extends to the regulation of sirtuins, particularly SIRT1 and SIRT3, which are critical for moderating cellular aging and stress responses. Activation of these proteins in stem cells has been linked to delayed senescence, improved differentiation capacity, and increased resistance to oxidative damage. In summary, melatonin helps maintain mitochondrial integrity and upholds the energy metabolism of stem cells, enabling them to make their way through complicated pieces of injury sites more effectively.
This relationship could be summarized through the following bullet list:
- Enhanced Mitochondrial Function: Melatonin improves ATP production and reduces reactive oxygen species.
- Activation of Sirtuins: Upregulation of key proteins such as SIRT1 and SIRT3 helps reduce cellular aging and promote differentiation.
- Improved Cell Viability: Crosstalk between melatonin and mitochondrial pathways increases the survival rate of transplanted cells.
Addressing the Tricky Parts of Stem Cell Delivery and Retention
One of the nerve-racking aspects of stem cell therapy is ensuring that the transplanted cells not only survive but also integrate effectively into the host tissue. The local ischemic niche is often loaded with problems ranging from extreme hypoxia to a lack of nutrients and inflammatory reactions. Under these conditions, even robust stem cells can struggle to survive.
Melatonin’s ability to protect against these issues is promising. Preconditioning stem cells with melatonin before transplantation helps them resist the influx of ROS and inflammatory mediators. This pre-treatment improves the retention time of the cells after transplantation, fostering a conducive environment for tissue vascularization and repair.
Despite these encouraging results, researchers continue to wrestle with some daunting issues:
- Cellular Retention: Even with melatonin pre-treatment, a significant proportion of cells are lost shortly after transplantation.
- Delivery Mechanisms: Establishing efficient delivery systems, such as melatonin-loaded nanoparticles, remains a technical challenge.
- Dosing Strategies: Determining the optimal melatonin concentration and exposure duration for different stem cell types is still a work in progress.
The use of advanced delivery vehicles—such as biodegradable nanoparticles that slowly release melatonin—has been investigated as a strategy to help smooth out some of these challenges. By protecting stem cells during the early critical period after transplantation, these approaches aim to significantly enhance long-term outcomes in tissue regeneration.
Melatonin’s Influence on Paracrine Signaling and Exosome Production
The healing process orchestrated by stem cells is not only due to their capacity to differentiate directly into vascular cells but also largely due to their paracrine signaling ability. Stem cells secrete extracellular vesicles, including exosomes, that carry microRNAs and proteins essential for tissue repair. Melatonin bolsters this process by enhancing the production and release of these vesicles.
Recent studies have shown that melatonin-treated stem cells release exosomes enriched with anti-inflammatory and pro-angiogenic factors. These exosomes help steer immune cells toward an anti-inflammatory phenotype, reducing local inflammation and further promoting the formation of new blood vessels.
This aspect of melatonin’s action represents a promising area of research, with potential applications not only in cardiac repair but also in treating diabetic wounds, renal injuries, and even spinal cord injuries. Here’s how melatonin influences exosome production:
- Immune Modulation: Exosomes help shift macrophages from an M1 (pro-inflammatory) to an M2 (anti-inflammatory) profile.
- Enhanced Angiogenic Factor Release: Melatonin increases the load of VEGF, bFGF, and related factors in exosomes.
- Reduction in Fibrosis: Exosomes aid in limiting scar tissue formation, thereby reducing long-term tissue dysfunction.
Lessons from Preclinical Studies and Future Directions
The current body of preclinical research illustrates melatonin’s multifaceted role in supporting stem cell therapy. Several animal studies have demonstrated that melatonin preconditioning of mesenchymal stem cells leads to improved outcomes following myocardial infarction, hindlimb ischemia, and other ischemic conditions. Tissue samples from experimental models treated with melatonin-preconditioned cells show enhanced microvascular density, reduced fibrotic lesions, and less overall tissue necrosis.
Despite these promising findings, the journey ahead is not without its twists and turns. There are still many confusing bits about the fine details of melatonin’s dose-dependent effects and the context in which its influence may become counterproductive. For instance, while moderate melatonin levels promote angiogenesis, higher doses might dampen the endothelial progenitor cells’ migration and the overall angiogenic response. These subtle parts of its action call for more research to identify not only the optimal dosage but also the right timing of administration.
Future studies need to focus on:
- Systematic dose-response trials in both in vitro and in vivo models to pinpoint the best concentration ranges.
- Longitudinal studies to evaluate the long-term retention and safety of melatonin-treated stem cells.
- Optimizing delivery systems, including controlled-release formulations to protect stem cells during the critical early phases after transplantation.
- Exploring the interactions between melatonin-induced exosomal signaling and immune regulation, particularly in diabetic and aging populations.
Researchers are also diving into the public databases to match gene expression profiles under melatonin treatment with improvements in angiogenesis outcomes. By using modern bioinformatics and molecular biology techniques, scientists are starting to decode the hidden complexities of melatonin’s action at the cellular level.
The Practical Implications for Regenerative Medicine
For clinicians and researchers in regenerative medicine, the application of melatonin in stem cell therapies is emerging as a critical improvement tool. The ease of availability, low toxicity, and multi-target potential of melatonin make it an attractive candidate for combination therapies. In clinics where patients suffer from ischemic conditions like myocardial infarction, dialysis for renal failure, or even diabetic foot ulcers, the adjunct use of melatonin could pivotally improve repair processes.
When integrating melatonin into therapeutic protocols, practitioners must consider several practical pointers:
- Patient-Specific Approaches: Every patient’s condition is unique. Tailoring the melatonin dosage based on individual oxidative stress levels and severity of the ischemic event can lead to better outcomes.
- Delivery Method: Whether administered systemically or used to precondition stem cells ex vivo, the choice of delivery must be optimized. Innovative drug delivery systems, such as encapsulated nanoparticles, offer exciting prospects for consistent melatonin release.
- Monitoring and Follow-Up: Continuous monitoring of angiogenic markers in patients can help clinicians adjust treatment plans to maximize tissue regeneration while minimizing adverse effects.
Table 1 below outlines some key considerations for incorporating melatonin into stem cell therapy protocols:
| Consideration | Description |
|---|---|
| Dosage Optimization | Determining the precise, patient-specific dose is super important to boost angiogenesis without dampening cell migration. |
| Delivery Mechanism | Exploring controlled-release systems and nanoparticle carriers for targeted melatonin delivery. |
| Preconditioning Protocols | Developing standardized protocols for melatonin pre-treatment of stem cells prior to transplantation. |
| Long-term Monitoring | Regular assessment of vascular density, cell retention, and tissue regeneration progress. |
Exploring the Controversial Aspects: Balancing Acts and Trade-Offs
Every innovative therapeutic approach comes with its set of trade-offs. For melatonin-enhanced stem cell therapies, one of the major controversial aspects is finding the right balance between its angiogenic and anti-angiogenic properties. In some experimental settings, melatonin has been observed to slow down chick embryo chorioallantoic membrane angiogenesis and reduce vascularization in certain types of diabetic complications.
This seemingly contradictory behavior underscores the need for a deep understanding of the little twists in its mechanism. The effect of melatonin may hinge on several small distinctions in experimental setup, such as the duration of exposure, the ambient oxygen levels, and even the specific cell types used. The current literature is loaded with studies that report both positive and negative outcomes, making it a tense area of research that calls for careful navigation.
A nuanced view of these conflicting pieces of evidence is necessary. When we poke around in the details, many reports suggest that while melatonin in moderate doses promotes angiogenesis, its high concentrations can suppress the proliferation of stem cells and inhibit critical signaling pathways like HIF-1α and STAT3. The key takeaway here is that the dosing protocol and the microenvironment conditions are super important to achieve the desired regenerative outcome.
Looking Ahead: Future Research and Therapeutic Horizons
While melatonin’s role in stem cell-based angiogenesis has shown exciting promise, several areas require further investigation before these therapies can be widely adopted in clinics. Future research should focus on the following aspects:
- Dose-Response Mechanisms: Detailed studies are needed to chart out the dose-response curves for various stem cell populations under different stress conditions.
- Advanced Delivery Systems: The development of smart delivery systems—such as melatonin-loaded hydrogels or nanoparticles—can ensure that the hormone is released gradually, fostering cell survival and integration.
- Longitudinal Clinical Studies: There is an essential need for long-term clinical trials to track the outcomes of patients receiving melatonin-preconditioned stem cell therapies, particularly in heart and kidney diseases.
- Mechanistic Insights: More detailed research into the molecular pathways modulated by melatonin could provide insights into how to fine-tune therapies to maximize benefits and minimize adverse effects.
The integration of state-of-the-art imaging, genomics, and proteomics will play a key role in these research endeavors. As translational medicine bridges the gap between bench and bedside, the collective efforts of biomedical researchers, clinicians, and bioengineers will be necessary to find your path through these tricky parts.
Moreover, collaborations across multiple disciplines and institutions could accelerate the pace of discovery and help standardize protocols that currently vary widely between research groups. In essence, while melatonin’s overall impact is promising, solving the tangled issues related to dosage, delivery, and cellular response is critical for transforming this potential into a practical therapy.
Final Thoughts: Weighing the Benefits Against the Challenges
Melatonin’s role in enhancing the angiogenesis potential of stem cells stands at the intersection of basic research and applied regenerative medicine. Its ability to protect stem cells from oxidative damage, boost their angiogenic secretions, and modulate mitochondrial and sirtuin activity makes it a super important adjunct in cell-based therapies. However, the road ahead is mixed with complicated pieces and nerve-racking questions that require carefully designed studies and clinical trials.
For now, the scientific community continues to dig into the promising aspects while remaining cautious of the potential pitfalls. Future therapies that integrate melatonin may well revolutionize the treatment of ischemic injuries and chronic cardiovascular diseases, providing patients with more robust and reliable tissue regeneration tools.
The journey to fully harnessing melatonin’s benefits is ongoing. As we work through the small twists in its mechanism and piece together the whole picture, one thing is clear: melatonin has reshaped our understanding of how a naturally occurring hormone can play a critical role in modern regenerative medicine. In this quest, the ability to find your way through the dose-dependent effects and complex signaling networks will be paramount.
Clinicians, researchers, and bioengineers alike have much to learn from ongoing studies and preclinical trials. While the road ahead is certainly layered with challenges, every step forward brings us closer to therapies that could one day transform how we treat some of the most overwhelming conditions affecting human health.
Key Takeaways in a Nutshell
To summarize, here are some of the key takeaways from the current findings on melatonin and stem cell angiogenesis:
- Protection from Oxidative Stress: Melatonin enhances the survival of stem cells by scavenging harmful free radicals.
- Boosted Angiogenic Secretions: Preconditioned stem cells release increased levels of VEGF and other growth factors that support new blood vessel formation.
- Mitochondrial Support: The hormone improves mitochondrial function and energy metabolism, facilitating cell repair.
- Dual-Action Phenomenon: Depending on the context, melatonin can either stimulate or inhibit angiogenesis, which underscores the need for careful dosing.
- Future Research Directions: Continued exploration into optimal dosing, delivery systems, and long-term outcomes is essential for clinical translation.
In closing, the potential of melatonin in regulating stem cell angiogenesis is a compelling frontier in the fight against cardiovascular and ischemic disorders. As the science matures and more nuanced insights emerge, it is crucial that we remain open to both the benefits and the challenges. Working through these tangled issues will be a group effort, and the cumulative findings will ultimately pave the way for next-generation regenerative therapies.
While there are many nerve-racking obstacles on the path to clinical application, the innovative spirit driving this research gives hope that future treatments will not only be safer but also more effective for patients who desperately need improved tissue repair and organ regeneration. The hope is that, with continued dedication, melatonin will prove to be the key to unlocking the full angiogenic potential of stem cells—ushering in a new era in regenerative medicine.
The integration of melatonin into stem cell therapy represents a promising blend of naturally occurring molecules and cutting-edge technology. With further research, we can expect refined protocols and drug delivery systems that make these therapies more robust, paving the way for significant advancements in patient care. The journey may be full of twists and turns, but each small step brings us closer to a future where regenerative medicine alleviates some of the most overwhelming health challenges of our time.
Originally Post From https://stemcellres.biomedcentral.com/articles/10.1186/s13287-025-04531-y
Read more about this topic at
Role of melatonin in the angiogenesis potential
Melatonin downregulates angiogenesis and …