Understanding BRCAness in Modern Oncology
BRCAness is an emerging concept in cancer research that has the potential to chart a new course for personalized cancer treatment. Essentially, BRCAness refers to certain traits in tumors—particularly those in ovarian, prostate, colon, pancreatic, and endometrial tissues—that exhibit defects in their DNA repair mechanisms, similar to cancers that arise from actual mutations in the BRCA1 or BRCA2 genes. Even when these mutations are absent, many tumors show similar tangled issues in their ability to repair double-stranded DNA breaks, paving the way for new treatment approaches that exploit these vulnerabilities.
This editorial takes a closer look at BRCAness, examining how these confusing bits in our genetic makeup can alter treatment strategies, broadening options for patients and clinicians alike. With treatments that target the defective repair machinery in cancer cells, doctors can more precisely take on tumors that might otherwise be resistant to conventional therapies. In this discussion, we will pore over the underlying science, explore the clinical benefits, and also address the many obstacles that make the identification and targeting of BRCAness a challenging but essential goal in modern healthcare.
What is BRCAness?
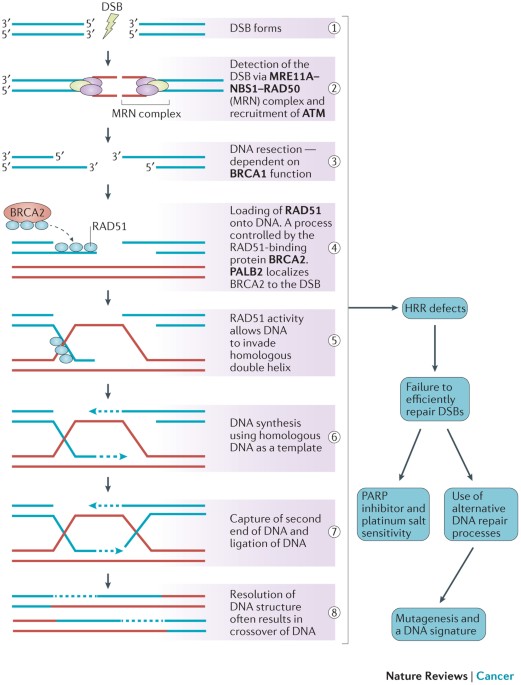
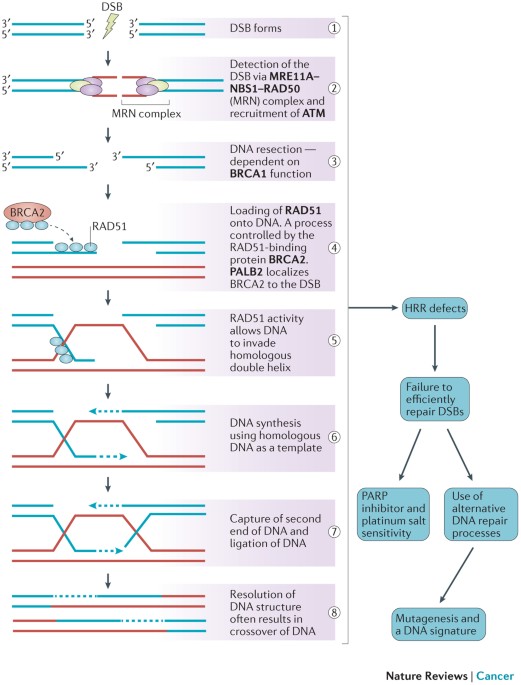
BRCAness is defined as the phenomenon where tumors—despite not having inherited mutations in the BRCA1 or BRCA2 genes—still display similar patterns of defective homologous recombination repair (HRR). Under normal circumstances, BRCA1 and BRCA2 play a key role in mending double-stranded DNA breaks with precision. When cells lose this repair capability—whether due to direct genetic mutations or because of secondary factors disrupting other repair pathways—the outcome can be a cancer-prone environment.
This idea, though initially a bit intimidating to researchers because of its many twists and turns, has reshaped the way clinicians think about targeted cancer therapy. Instead of limiting treatment to those with known genetic alterations, BRCAness opens up the possibility of treating a wider range of patients by identifying similar repair defects in tumors that do not carry BRCA mutations. In other words, the presence of BRCAness means that the tumor may be sensitive to treatments that generate DNA damage, such as platinum-based chemotherapies and poly (ADP-ribose) polymerase inhibitors (PARPi).
Defining the DNA Repair Challenge
Most of us know that our cells are constantly exposed to agents that can damage DNA. Radiation, smoking, and even natural metabolic processes can introduce double-strand breaks. The repair of these breaks is not only critical but also must be done with precision. A healthy cell uses homologous recombination repair (HRR), a system that depends on the proper functioning of BRCA1, BRCA2, and other companion proteins. However, when these proteins are compromised, the repair process gets tangled with error-prone alternatives like non-homologous end joining (NHEJ), leading to additional mutations and a possible increase in cancer risk.
This dependence on a finely tuned repair system is a fine example of how the hidden details in our DNA can have large consequences. When we consider BRCAness, we see that even in the absence of the well-known BRCA mutations, tumors can have deoxyribonucleic acid (DNA) repair defects that create similar opportunities for targeted intervention. It’s like discovering that a house built with a specific set of materials still faces the same structural flaws even if it doesn’t use the expected type of wood. As a result, the strategies to address these problems can be broadened to include a larger group of patients.
DNA Repair Mechanisms and Their Impact on Cancer
The human body relies on a host of repair strategies to safeguard the genetic code. The homologous recombination repair system is one of the key repair mechanisms, ensuring that the DNA damage accumulated during everyday cellular activities is correctly fixed. However, when there is a failure in this system—either because of a direct mutation in the BRCA genes or due to malfunctioning in other related repair genes—the damage accumulates and could ultimately lead to cancer.
How HRR Works: The Nitty-Gritty of DNA Fixing
Under normal cell conditions, whenever a double-strand break occurs, the cell responds by setting up a repair crew. The BRCA proteins help create structures—often called RAD51-single-stranded DNA (ssDNA) filaments—that are crucial for finding the correct template and mending the break. This process is not only finely tuned but is also most effective during the phases of the cell cycle when a sister chromatid is available, typically during S and G2 phases.
Once the break is noticed, the cell meticulously aligns the damaged section with its undamaged counterpart. If this process is undermined—whether by mutations in BRCA1/2 or other HRR-related genes such as RAD51C, PALB2, or BRIP1—the reliability of DNA repair is compromised. Without this perfect match-up, cells are forced to adopt more error-prone methods like non-homologous end joining, which can introduce unintended mutations. These problematic mutations can then accumulate, increasing the chance of transformation into cancer.
Comparing Standard BRCA Mutations to BRCAness Traits
| Characteristic | BRCA Mutations | BRCAness Traits |
|---|---|---|
| Genetic Alteration | Direct mutations in BRCA1/2 genes | Mutations or epigenetic changes in HRR-related genes (e.g., RAD51C, PALB2, BRIP1) |
| DNA Repair Capability | Impaired homologous recombination repair | Similar repair deficiencies despite lack of BRCA mutations |
| Tumor Sensitivity to Treatment | Responsive to platinum drugs and PARPi | Exhibit vulnerabilities to similar DNA damaging agents |
| Screening Focus | Genetic testing for BRCA mutations | Comprehensive genomic profiling to detect subtle repair defects |
This table helps underscore the similarities and differences between classical BRCA mutations and the broader concept of BRCAness. Both scenarios result in a reduced capacity for precise DNA repair, thereby increasing the tumor’s susceptibility to treatments that work by damaging DNA further.
Targeted Therapies and the Promise of Personalized Treatment
One of the most exciting prospects in modern oncology is the tailored approach to treating cancer. By working through the tangled issues of individual tumor biology, clinicians are increasingly able to pick treatments that target the specific vulnerabilities of a patient’s cancer. BRCAness is a shining example of this trend, as it allows for therapies designed to exploit the defective DNA repair machinery found in various tumors.
PARP Inhibitors: Exploiting DNA Repair Defects
Poly (ADP-ribose) polymerase inhibitors, better known as PARPi, have become a cornerstone in the treatment of cancers that show signs of impaired HRR. By blocking the PARP enzyme—which plays a role in repairing single-strand breaks—these drugs create a situation of synthetic lethality. For tumors that already have weakened defenses due to BRCAness, this additional inhibition of the repair pathway leads to a buildup of unrepaired DNA damage, eventually forcing the cancer cells to perish.
Clinical trials have demonstrated that cancers expressing BRCAness traits, including some glioblastomas and ovarian tumors, often have better responses to PARPi therapy. Even if these patients do not carry the classic BRCA mutations, their tumors can still be remarkably sensitive to the DNA-damaging effects of such targeted treatments. This outcome is a testament to the value of carefully probing the hidden details of a tumor’s genetic makeup—allowing doctors to figure a path through the maze of cancer biology and select a treatment that promises the best chance of success.
Platinum-Based Chemotherapies: Enhancing Treatment Efficacy
Another class of agents that benefits from the concept of BRCAness is platinum-based chemotherapy. Drugs like cisplatin and carboplatin cause cross-links in DNA strands, preventing the proper unwinding and replication of the genetic code. For tumors already struggling under the weight of defective HRR, these treatments can be particularly effective.
The attractiveness of platinum-based chemotherapy lies in its ability to generate substantial DNA damage, overwhelming the limited repair capacity in cells exhibiting BRCAness. This targeted approach results in prolonged treatment responses and, in many cases, improved patient survival. It is the culmination of years of research into the tiny details of DNA repair that now allows patients with otherwise hard-to-treat cancers to benefit from these therapies.
Overcoming the Tricky Parts of Diagnosing BRCAness
Despite the promise offered by therapies targeting BRCAness, identifying this trait in tumors is not without its nerve-racking challenges. The process involves intricate genomic profiling, which must accurately identify mutations, epigenetic alterations, and other subtle changes in a tumor’s HRR machinery. This is a daunting task, given the confusing bits and twists and turns seen in complex cancer biology.
Diagnostic Techniques: Gene Panels and Functional Assays
One of the main hurdles in detecting BRCAness lies in the tools currently at our disposal. Gene sequencing panels, such as FoundationOne CDx, screen hundreds of cancer-associated genes, but they often stumble when it comes to distinguishing between pathogenic mutations and benign alterations. In addition, functional tests—like assessing RAD51 foci formation as an indirect measure of HRR proficiency—remain in the experimental stage and are not yet standardized across clinical settings.
To further complicate matters, tumors are full of problems when it comes to heterogeneity. Not only can the gene expression vary significantly from one section of a tumor to another, but factors in the microenvironment can also play a role in masking the underlying deficiencies. This heterogeneity requires a multi-faceted approach to diagnosis, combining genomic data with functional assays and even histological assessments to build a comprehensive picture of the tumor’s repair capabilities.
Bulleted List of Diagnostic Challenges:
- Genomic Heterogeneity: Variations within the tumor can hide true repair defects.
- Overlapping Functions: Many genes share tangled issues in the repair process, making it hard to pinpoint one specific defect.
- Epigenetic Factors: Changes such as promoter hypermethylation can mimic genetic mutations, adding extra layers for analysis.
- Reliability of Functional Assays: Tests like RAD51 foci measurement are not yet reliable enough for routine clinical use.
- Standardization of Testing Protocols: The lack of a universally accepted diagnostic protocol makes it challenging to compare patient data across different centers.
Each of these points underscores the delicate nature of pinpointing BRCAness. It is a process that requires both technological innovation and collaborative standardization among laboratories and clinicians. The payoff, however, would be a clearer identification of patients who might benefit from therapies that are tailored to their tumor’s specific repair shortcomings.
Future Directions: Advancing Personalized Cancer Care
The concept of BRCAness not only enriches our understanding of tumor biology but also has the potential to revolutionize the way we approach cancer treatment. As research digs deeper into the many twists and turns of DNA repair pathways, it becomes increasingly clear that personalized treatment strategies can yield exceptional outcomes for patients with varied types of tumors.
Integrating Genomic and Functional Data
Looking ahead, one of the key strategies will be to integrate comprehensive genomic profiling with functional assays that measure the real-time competence of the DNA repair machinery. This integrated approach would create a more detailed and actionable profile of a tumor’s repair status. For clinicians, this means having the tools to precisely figure a path through the conflicting signals given by genetic data and arrive at a treatment plan that is both individualized and highly effective.
To achieve this, multidisciplinary teams comprising oncologists, molecular biologists, and bioinformaticians must work together. They need to refine the definitions of what constitutes BRCAness and develop more reliable assays that can be seamlessly incorporated into clinical practice. By shining a spotlight on these hidden complexities, the field can move away from a one-size-fits-all treatment model and instead offer personalized care that works on the level of the individual tumor’s biology.
Emerging Technologies and Biomarker Discovery
Technological innovations will also have a crucial role to play. Advances in high-throughput sequencing, machine learning, and single-cell analysis are already starting to unmask the detailed picture of how tumors function at the molecular level. These tools can help identify subtle patterns in genetic expression and methylation that were previously obscured by the overall complexity of cancer biology.
A particularly promising development is the use of RNA sequencing and transcriptional profiling, which can provide insights into the small distinctions and fine shades of gene expression that define BRCAness. Similarly, evaluating promoter methylation patterns offers another layer of detail that could be critical in understanding which tumors are most likely to respond to PARPi or platinum-based therapies.
Collaborative Clinical Trials and Research Initiatives
No medical advancement is made in isolation. Collaborative trials that bring together experts from multiple research institutions are essential to validate new biomarkers and treatment strategies. Recent studies involving PARP inhibitors like olaparib and niraparib have shown that even patients without BRCA mutations can experience substantial benefits if their tumors exhibit BRCAness. Such collaborative efforts will be the key to overcoming the tricky parts and nerve-racking challenges of diagnosing and treating these tumors.
For these trials to be successful, a few key elements must be in place:
- Standardized Diagnostic Protocols: Developing a consensus on testing methods will allow clinicians to compare data across different studies and patient populations.
- Comprehensive Data Sharing: Collaborative networks where researchers exchange data can accelerate the discovery process.
- Patient-Centered Approaches: Ultimately, the most important aspect is how these findings translate into improved patient care and survival rates.
Examining the Broader Implications of BRCAness in Oncology
BRCAness not only transforms treatment possibilities for cancers traditionally associated with BRCA mutations but also helps to extend these advanced therapies to other challenging tumor types. The idea is that by taking a closer look at each tumor’s defective DNA repair system, doctors can figure a path through the maze of treatment options and select strategies that are most likely to yield positive results.
The Ripple Effect on Cancer Care
The potential benefits of understanding BRCAness reach far beyond the treatment of individual patients. As this area of research grows, it sets the stage for a broad shift in how oncology approaches cancer therapy. Key points include:
- Wider Patient Eligibility for Targeted Therapies: More patients may become eligible for PARPi and platinum drugs, even if they lack classic BRCA mutations, thanks to the identification of BRCAness traits.
- Optimized Treatment Protocols: Tailored therapies mean that treatments can be better timed and dosed to match the tumor’s repair profile, potentially reducing side effects and improving efficacy.
- Enhanced Prognostic Indicators: A detailed understanding of a tumor’s repair capacity can help predict how aggressively the cancer might behave and how well it might respond to certain treatments.
- Cost-Effective Treatment Planning: By targeting treatments more precisely, healthcare systems can avoid the trial-and-error approach, saving both time and resources.
Such ripple effects emphasize not only the clinical importance of identifying BRCAness in tumors but also its potential economic and quality-of-life impacts. When treatments are tailored to individual tumor biology, patients are more likely to receive effective care with fewer unnecessary interventions.
Maintaining a Balanced Perspective on BRCAness
While the promise of BRCAness is enormous, it is equally important to maintain a neutral perspective and acknowledge the many tricky parts that remain. There is still a great deal that researchers need to figure out about the exact mechanisms that lead to these repair deficiencies in tumors. Much of the current work is loaded with issues related to tumor heterogeneity, the reliability of diagnostic tests, and the overall standardization of detection methods.
Balancing Optimism with Caution
It is easy to get excited about potential breakthroughs, but caution remains necessary. The field is still in the process of grappling with several confusing bits about the nature of BRCAness. These include:
- Determining the precise role of alternative DNA repair pathways
- Understanding how epigenetic modifications interact with genetic mutations to produce these traits
- Addressing the challenges posed by tumor heterogeneity, which can obscure clear readings of a tumor’s repair status
Given these issues, clinicians and researchers must dig into the problem with a balanced approach—embracing the potential benefits of new targeted therapies while continuing to work through the many layered, problematic details of DNA repair in cancer cells.
The Role of Policy and Funding in Advancing Research
The progress towards integrating BRCAness into routine cancer care also depends significantly on broad support from policy makers and funding agencies. Adequate funding for collaborative research initiatives and standardization projects is essential. When governments and private entities invest in high-quality studies, the pace of innovation accelerates, leading to more refined treatments and better patient outcomes.
In addition, regulatory frameworks that support the rapid translation of laboratory findings into clinical settings can shorten the gap between discovery and treatment implementation. Policymakers must recognize the high stakes involved in treating aggressive and often resistant cancers, and work to create an environment where scientific advances can move quickly from the bench to the bedside.
Charting a Course for the Future
The concept of BRCAness represents a paradigm shift in cancer care. It offers a means of personalizing therapy to an extent that was once thought to be beyond reach. By understanding and exploiting the defective DNA repair mechanisms that many tumors share, a wider array of patients can be offered treatments that are more effective and tailored to their unique cancer profile.
Personalized Cancer Treatment: Real-World Benefits
What does this mean for patients? In practical terms, identifying BRCAness allows for:
- More Inclusive Treatment Options: Patients who might previously have been excluded from certain treatments due to lack of BRCA mutations can now benefit from targeted therapies.
- Improved Response Rates: By matching the therapy to the tumor’s specific repair defect, response rates to treatments like PARPi and platinum-based drugs are significantly enhanced.
- Longer Disease-Free Intervals: Clinical studies have shown that patients with tumors exhibiting BRCAness often enjoy longer periods without disease progression.
- Better Overall Survival: Ultimately, the goal is to increase survival rates and improve the quality of life for cancer patients, making this research not merely academic but a critical component of modern oncology.
By taking a closer look at the DNA repair capacity of tumors, doctors can make more informed decisions, ensuring that each patient receives the most appropriate treatment for their specific condition. Personalized treatment strategies built on a foundation of robust genomic and functional data can revolutionize the patient experience, offering hope where traditional therapies might have fallen short.
Next Steps in Research and Clinical Practice
The continued success of personalized cancer care through the lens of BRCAness depends on several immediate next steps:
- Refinement of Diagnostic Tools: Investing in research that aims to fine-tune diagnostic assays and sequencing platforms is essential. With better instruments, the false negatives and positives that currently plague testing can be significantly reduced.
- Standardized Protocols: Developing universally accepted guidelines will allow clinicians to compare and share patient data more effectively. This standardization is a crucial step in ensuring that every patient, regardless of geographical location, has access to the best diagnostic and treatment options.
- Cross-Disciplinary Collaboration: Scientists, clinicians, bioinformaticians, and policymakers must work in concert to uncover the little twists and subtle details of BRCAness. Together, they can create a unified strategy that maximizes patient benefit and minimizes trial-and-error approaches.
- Long-Term Clinical Trials: More extended studies that track patient outcomes over many years are needed to fully understand the benefits and limitations of using BRCAness as a treatment guide. Only then can its real-world efficacy be confirmed and optimized.
These next steps are not without challenges, but the potential payoff is enormous. By methodically untangling the complicated pieces of DNA repair defects and their clinical implications, the oncology community can lay the groundwork for a future where cancer treatment is both personalized and highly effective.
Conclusion: A New Era in Cancer Care
In closing, the concept of BRCAness offers a transformative way to look at cancer treatment. Rather than being limited by the presence or absence of specific gene mutations, clinicians can now consider the broader picture of DNA repair deficiencies. This approach has been embraced by clinical trials exploring PARPi and platinum-based therapies, with compelling evidence suggesting markedly improved outcomes for patients whose tumors exhibit these defective repair traits—even in the absence of BRCA mutations.
While there are still many nerve-racking and overwhelming challenges to tackle in making BRCAness a standard part of cancer diagnosis and treatment, the potential benefits are too significant to ignore. As research continues to poke around into the hidden complexities of the DNA repair process, there is growing optimism that these insights will lead to more inclusive, effective, and personalized treatment strategies for a wide range of cancers. It is an exciting time in oncology as the integration of genomic and functional data, along with advanced diagnostic techniques, promises to reshape patient care.
Ultimately, the journey of BRCAness—from a scientific observation to a cornerstone of personalized therapy—exemplifies the innovative spirit that drives modern medicine. With continued collaboration, improved diagnostics, and robust clinical trials, the future of cancer treatment is set to become more adaptable, precise, and tailored to the individual needs of patients. This progress not only enhances the quality of care but also brings us a step closer to managing one of medicine’s most challenging diseases with compassion and cutting-edge science.
It is our responsibility as clinicians, researchers, and policymakers to steer through these tricky parts and create a healthcare landscape where every patient has the opportunity to benefit from these targeted treatments. BRCAness, with all its tangled issues and subtle indicators, symbolizes hope—a beacon guiding us toward a future of truly personalized cancer care.
Originally Post From https://www.news-medical.net/health/Understanding-BRCAness-What-It-Means-for-Cancer-Treatment.aspx
Read more about this topic at
Precision or Personalized Medicine
What Is Precision Cancer Medicine? | The AACR