Groundbreaking Immunotherapy: Car T-Cell Therapy for Solid Tumours
The recent clinical trial results from China have sparked an invigorating debate in the field of oncology. Researchers have demonstrated that Car T-cell therapy, which involves genetically modifying a patient’s own white blood cells to target cancer, has enabled patients with advanced gastric and gastro-oesophageal junction (GEJ) cancer to live, on average, approximately 40% longer than those receiving standard treatments. This achievement is more than a scientific milestone—it is a tangible step forward in addressing some of the most tricky parts of treating solid tumours.
Car T-cell therapy has traditionally been successful in treating blood cancers, but its application to solid tumours represents new territory filled with both promise and challenges. In this opinion editorial, we will take a closer look at the significance of these findings, explore the potential hurdles, and dig into what the future may hold for this innovative treatment.
Advanced Gastric Cancer Immunotherapy Options
In a recent randomised controlled trial, over 100 patients with advanced gastric or GEJ cancer were assigned to receive either Car T-cell therapy or standard-of-care medications. Reports indicate that those treated with the designer immunotherapy experienced an average survival of 7.9 months, compared to 5.5 months for those on conventional treatments.
The study also revealed that patients treated with Car T-cell therapy enjoyed a mean progression-free survival of 3.3 months, nearly double that of the standard care group, who averaged 1.8 months. Such improvements highlight the clinical relevance of this approach for patients with limited treatment options, offering a ray of hope amid a landscape loaded with challenges.
Key Highlights of the Study
- Improved Overall Survival: An average increase of 40% in survival time.
- Longer Progression-Free Survival: Patients experienced over 3 months before their cancer advanced compared to under 2 months with standard care.
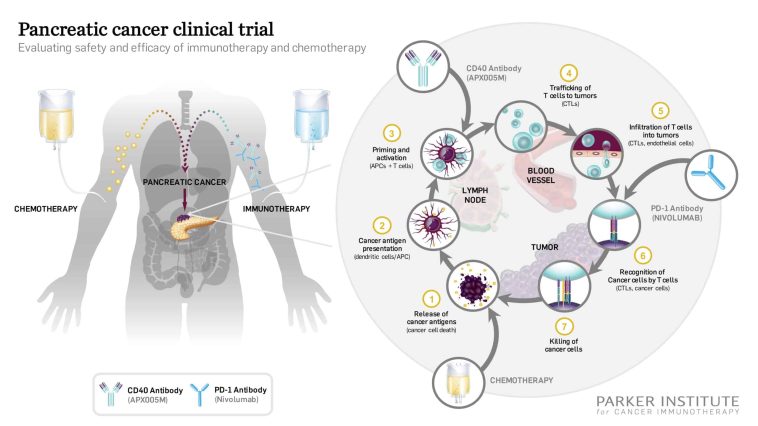
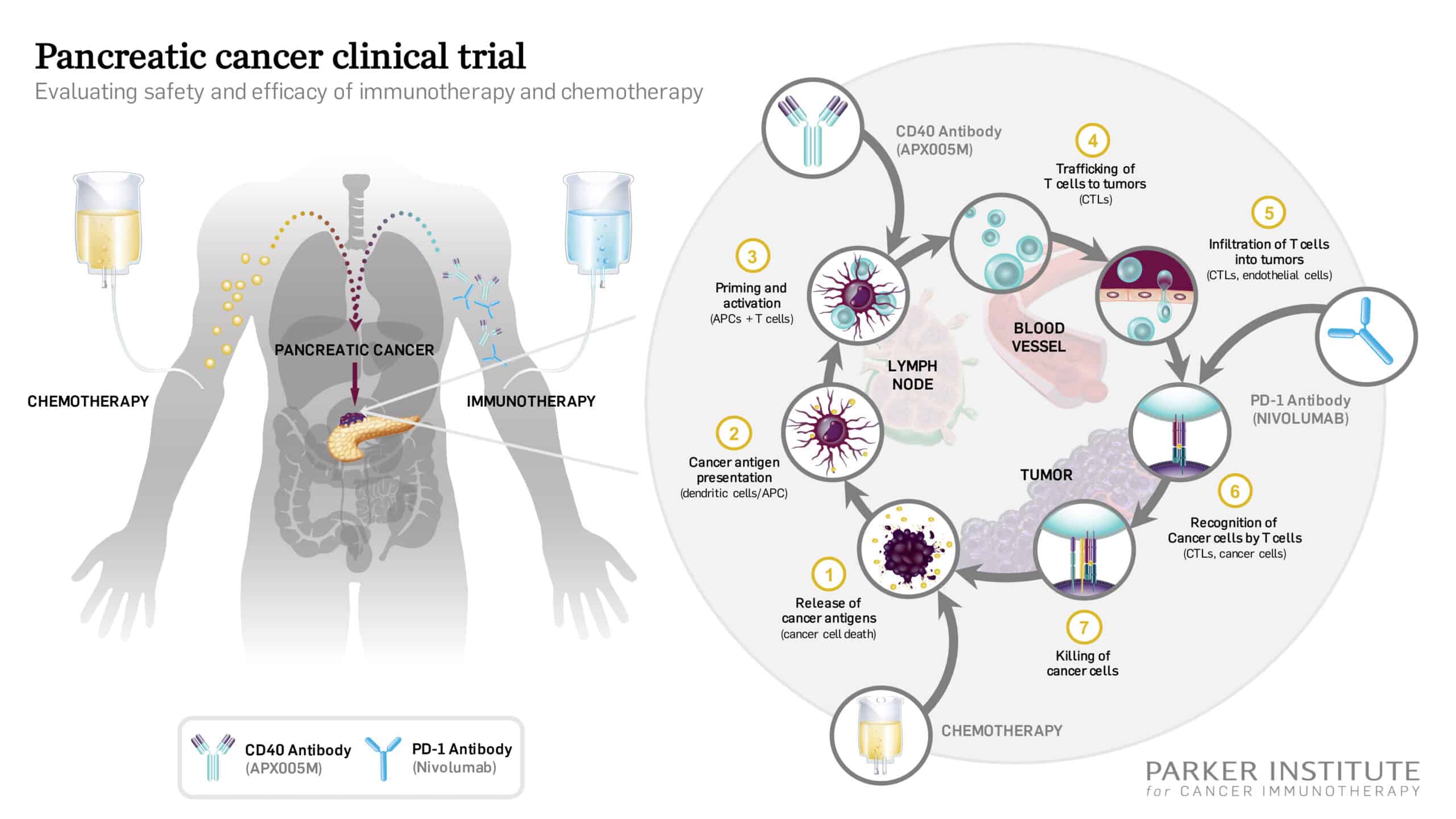
- Targeted Therapy: Use of genetically engineered T-cells to specifically attack cancer cells.
- Worldwide Impact: Although the trial was based in China, its implications are global and underscore the potential for broader application.
These highlights not only showcase the advancements in treatment options but also point toward a future where such therapies could become essential in managing solid cancers like breast, lung, and pancreatic cancers, which together account for nearly 90% of all cancer cases.
Innovative Car T-Cell Therapy for Solid Tumour Treatment
While the promise of Car T-cell therapy is bright, the path is filled with nerve-racking twists and turns. The technology that reprograms the body’s T-cells to recognise and combat cancer represents a shift from traditional cancer treatments and introduces new ways of steering through the treatment process.
One of the significant advantages of Car T-cell therapy in solid tumours is its ability to overcome some of the tricky parts associated with treating these cancers. Solid tumours often have a microenvironment that is not only complicated but also off-putting, presenting numerous small distinctions that make treatment particularly challenging. The genetically modified cells are designed to home in on cancer cells with an accuracy that conventional drugs may struggle to replicate.
The Science Behind Car T-Cell Engineering
The process of creating Car T-cells involves:
- Harvesting T-cells: Extracting a patient’s own T-cells from their blood.
- Genetic Modification: Reprogramming the T-cells in a laboratory to express a specific chimeric antigen receptor (Car) that can detect cancer cells.
- Expansion and Infusion: Multiplying the modified cells and reinfusing them into the patient’s bloodstream.
A simplified table summarises the steps:
| Step | Description |
|---|---|
| T-cell Collection | Harvesting the patient’s white blood cells through a blood draw. |
| Genetic Reprogramming | Using viral vectors to insert the gene encoding the targeted receptor. |
| Cell Expansion | Growing the modified cells in a controlled laboratory environment. |
| Reinfusion | Infusing the engineered cells back into the patient to attack the tumour. |
By transforming T-cells into precision tools that can identify and kill cancer cells, this therapy attempts to tackle the hidden complexities of cancer’s evasion techniques. Still, the road ahead is not without its tricky bits, and further research is crucial in refining the approach and expanding its use.
Patient Experience and Quality of Life Considerations
One critical aspect of any new therapy is its impact on the patient’s day-to-day life. With Car T-cell therapy, patients not only show improved survival figures but also enjoy a longer period where the cancer does not progress. This progression-free period is essential for maintaining a patient’s quality of life, allowing them more time to enjoy daily activities and spend precious moments with loved ones.
The trial results suggest that the modified T-cells may better manage the tangled issues related to controlling tumour growth. Patients with advanced gastric cancers, who typically face off-putting treatment regimens, are now presented with an option that may give them back time and a better quality of life.
Benefits of Extended Progression-Free Survival
- Emotional Relief: Patients experience less frequent hospital visits and treatments, reducing medical stress.
- Physical Well-Being: Fewer side effects can be expected compared to traditional chemotherapy.
- Improved Daily Function: A longer period without tumour advancement allows an improved ability to perform routine tasks.
- Family Time: With extended survival times and fewer treatments, patients can create more memories with their families.
While these advantages are promising, medical professionals caution that rigorous monitoring and larger clinical trials are necessary to confirm the broader applicability of Car T-cell therapy. The tricky parts of balancing efficacy, side effects, and cost must be addressed to ensure that this therapy can be safely and widely adopted.
Exploring the Challenges and Risks Involved
Even as the clinical trial results are hailed as a breakthrough, the hurdles in applying Car T-cell therapy to solid tumours are loaded with issues that require closer analysis. The nature of solid tumours—with their protective microenvironments and varied biological characteristics—introduces complicated pieces that mean the success rate and potential side effects can vary widely among patients.
Some of the possible risks and limitations include:
- Cytokine Release Syndrome (CRS): A potentially severe immune response where the infused T-cells cause a rapid release of cytokines, leading to high fevers and flu-like symptoms.
- Neurological Complications: Patients may experience confusion, seizures, or other neurologic symptoms as a result of immune activation.
- On-Target Off-Tumour Toxicity: Genetically modified T-cells might attack not only cancer cells but also healthy cells that share similar markers, causing unintended damage.
- Cost and Accessibility: The production process for Car T-cells is expensive and complex, potentially limiting access for many patients.
Managing the Risks: A Closer Look
Medical teams need to be prepared to quickly respond to the nerve-racking side effects that might occur with Car T-cell therapy. To illustrate how these challenges are being managed, consider the following strategies:
- Early Detection and Intervention: Monitoring patients closely during and after therapy to catch complications early.
- Customized Dosing: Adjusting the therapy based on patient-specific factors to minimise adverse reactions.
- Supportive Care: Providing medications and treatments alongside Car T-cells to manage symptoms like inflammation and CRS.
The table below summarises some of the key strategies for risk management in Car T-cell therapy:
| Risk | Management Strategy |
|---|---|
| Cytokine Release Syndrome | Pre-treatment with anti-inflammatory drugs, careful dosing, and supportive care. |
| Neurological Complications | Continuous neurological monitoring and rapid intervention with steroids if needed. |
| On-Target Off-Tumour Toxicity | Enhanced screening of target markers and adjustment of T-cell specificities. |
| Cost and Scalability | Investment in new manufacturing technologies to reduce production costs. |
These approaches, while promising, underscore the fact that the application of Car T-cell therapy in a solid tumour setting is both exciting and on edge. The hidden complexities and fine points of genetic engineering and cancer biology require not only scientific innovation but also practical, hands-on adjustments as the treatment is refined.
Comparing Car T-Cell Therapy with Conventional Cancer Treatments
Traditional cancer treatment methods, such as chemotherapy and radiotherapy, have been the standard for decades. However, they often come with overwhelming side effects and do not specifically target cancer cells, sometimes harming healthy tissues along the way. In contrast, Car T-cell therapy represents the future of personalised medicine—tailored treatments that actively engage the body’s immune system in the fight against cancer.
By comparing these two approaches, it becomes clear that while conventional therapies continue to play a critical role in cancer management, the Car T-cell method offers a more precise mode of action. This level of exactness is achieved by teaching T-cells to monitor the body closely and zero in on cancer cells with impressive accuracy, thereby reducing collateral damage.
Pros and Cons: A Comparative Overview
- Precision Targeting: Car T-cell therapy is highly specific, reducing damage to normal cells, whereas chemotherapy is more generalized.
- Side Effects: Although Car T-cell therapy can trigger immune reactions like CRS, the overall side-effect profile may be less severe than the systemic toxicity of chemotherapy.
- Personalization: Car T-cell therapy leverages the patient’s own cells, which can translate to a more personalised treatment protocol compared to one-size-fits-all chemotherapy regimens.
- Production Complexity: The process of creating genetically modified cells is an intricate procedure, unlike the mass production of chemical treatments, presenting unique logistical and cost challenges.
A visual summary can be seen in the table below:
| Aspect | Car T-Cell Therapy | Conventional Treatments |
|---|---|---|
| Treatment Specificity | High, with targeted action against cancer cells | Low to moderate, affecting both cancerous and healthy cells |
| Side-Effect Profile | Potential for immune reactions, but focused | Systemic toxicity and numerous side effects |
| Personalization | Tailored based on individual genetic and immune profiles | Generally uniform across patients |
| Production and Cost | Complex and expensive; requires cutting-edge technology | More cost-effective with standardized protocols |
This comparative overview clearly indicates that while Car T-cell therapy shows immense potential, it must undergo further refinement and broader testing to become a widespread alternative to traditional cancer treatments.
Future Perspectives: The Road Ahead for Car T-Cell Therapy
The encouraging findings from the trial in China are just the beginning. Experts believe that with further research, modified Car T-cell strategies could be adapted for various solid tumours, including notoriously aggressive cancers like glioblastoma in the brain. New studies already underway are expected to shed light on the therapy’s benefits in managing brain tumours, potentially extending its reach to patient groups in desperate need of new treatment options.
Looking ahead, the success of Car T-cell therapy in solid tumours could represent a paradigm shift in oncology. The innovative treatment not only stretches survival times but also promises improved quality of life for patients who have exhausted other treatment avenues. However, fully realising this promise requires additional trials, a deeper understanding of the subtle parts of tumour biology, and technological advances that make the therapy less intimidating and more accessible to all patients.
Emerging Research and Next Steps
Current and future research initiatives are focusing on several key areas to further enhance the safety and efficacy of Car T-cell therapy:
- Expanding Clinical Trials: Larger, multi-centre studies will help determine the optimal dose, timing, and combination with other treatments.
- Combination Therapies: Researchers are exploring how Car T-cell therapy can be integrated with other modalities like checkpoint inhibitors to overcome the protected microenvironment of solid tumours.
- Biomarker Development: Identifying reliable biomarkers to predict which patients are most likely to respond to the therapy.
- Cost Reduction Strategies: Investing in technology and improved manufacturing processes to bring down the overall costs and make the treatment more widely available.
A forward-looking table can aid in visualising these future steps:
| Focus Area | Objective |
|---|---|
| Clinical Expansion | Conduct larger trials with diverse patient populations to validate early results. |
| Combination Strategies | Explore synergies between Car T-cell therapy and other advanced treatments. |
| Biomarker Discovery | Develop tests to pinpoint patients who would benefit most from the therapy. |
| Cost Efficiency | Streamline production processes and invest in innovative production platforms. |
As research continues to dig into the hidden complexities of cancer treatment, the next decade could see Car T-cell therapy evolve into a standard care approach, especially in solid tumour cases that have, until now, been difficult to treat with precision.
Personalized Medicine and the Future of Oncology
The transformation underway in cancer treatment is a testament to the power of personalised medicine—an approach that promises therapies crafted to fit a patient’s unique genetic and immunological profile. Car T-cell therapy embodies this philosophy by effectively retooling a patient’s own immune system to combat the disease head-on.
With the integration of genomics, proteomics, and data analytics in healthcare, the modern treatment landscape is rapidly shifting. This means that the fine points of patient selection, therapy customisation, and real-time monitoring are becoming super important in clinical practice. Making your way through these developments requires collaboration between clinicians, researchers, and policy-makers to ensure that innovative treatments such as Car T-cell therapy are not only effective but also accessible to all who need them.
Impact on Personalized Treatment Strategies
Some of the key benefits of embracing this personalized approach include:
- Enhanced Efficacy: Tailoring treatments to individual patients can improve therapy outcomes and minimize unnecessary side effects.
- Greater Safety: By selecting patients most likely to benefit, doctors can reduce the risk of severe reactions and improve overall care.
- Improved Research Focus: Personalised data enables researchers to identify effective combinations and refine protocols in real time.
These factors converge to create a treatment paradigm that moves away from one-size-fits-all methods toward more precise, data-driven care. Notably, Car T-cell therapy is at the forefront of this shift, demonstrating how genetic reprogramming and immune system manipulation can come together to produce outcomes that were once considered beyond reach.
Socioeconomic Considerations and Accessibility of Advanced Treatments
Despite its revolutionary potential, Car T-cell therapy brings with it a set of challenging socioeconomic questions. As with many cutting-edge treatments, the cost and accessibility of Car T-cell therapy remain critical factors in determining its overall impact. While early results are promising, healthcare systems around the world must find ways to make such treatments accessible, especially to those who could benefit the most.
Key aspects to consider include:
- Cost-to-Benefit Ratios: Balancing the high production and administration costs with the benefits in survival and quality of life.
- Insurance and Policy Frameworks: Updating healthcare policies to incorporate reimbursement for advanced therapies.
- Global Accessibility: Ensuring that breakthroughs in treatment can reach patients worldwide, not just in well-funded healthcare systems.
A comparative cost analysis can help stakeholders figure a path toward widespread adoption of such therapies:
| Factor | Traditional Treatments | Car T-Cell Therapy |
|---|---|---|
| Cost | Generally cost-effective with established manufacturing | High initial costs due to personalised cell engineering |
| Access | Widely available | Limited to specialised centres at present |
| Efficacy | Variable; may involve significant side effects | High specificity with promising survival improvements |
Addressing the socioeconomic challenges requires a multidisciplinary effort—spanning government policy, healthcare provider initiatives, and pharmaceutical advancements. Only by working through these tricky bits can the promise of Car T-cell therapy be fully realised for patients across the globe.
Expert Opinions and the Collective Voice of the Medical Community
The broader oncology field is buzzing with discussions about the potential of Car T-cell therapy. Leading experts, armed with a wealth of clinical experience, have expressed cautious optimism regarding the trial’s results. Notable voices in the medical community appreciate that while the trial is a major step forward, it is also a call to further investigate the subtle details and little twists associated with the new treatment approach.
Experts like Dr. Carl June and Dr. Jason Luke have highlighted the transformative nature of Car T-cells in treating both blood cancers and, now, solid tumours. According to their assessments, the evidence emerging from these initial trials should inspire the cancer research community to intensify efforts in refining the approach and expanding its application.
Insights from the Oncology Community
Some notable expert opinions include:
- Optimism with Caution: The improvements in progression-free survival and overall survival are exciting, yet further trials are essential before broad implementation.
- Challenges of Scaling: Experts are very much aware of the off-putting, complicated pieces involved in mass-producing such personalized therapies.
- Collaborative Approach: There is a consensus that collaboration between research institutions worldwide is a must-have to iron out the finer, smaller distinctions in treatment protocols.
This collective voice underscores the importance of remaining neutral while embracing the potential for change. As with any leap forward, balancing the promise of greatly extended life spans with the inherent risks associated with such revolutionary treatments is a critical task for the future of healthcare.
Concluding Thoughts: A New Era in Cancer Treatment?
In conclusion, the emerging evidence from clinical trials of Car T-cell therapy in solid tumours represents a significant departure from conventional oncology treatments. The extension of survival times by approximately 40% in patients with advanced gastric and GEJ cancers is a promising development that warrants further exploration, research, and investment.
The road ahead is filled with both promising breakthroughs and nerve-racking challenges. Moving forward, the following points stand out as super important:
- Expand clinical trials to larger, more diverse populations.
- Improve manufacturing processes to reduce costs and improve accessibility.
- Integrate this therapy with other emerging treatments to maximize outcomes.
- Maintain a focus on personalized patient care to address individual diagnostic and therapeutic needs.
As we figure a path through the uncertain future of cancer treatment, it is essential that the academic community, clinicians, and policymakers work together. The fine shades of each new development must be scrutinized carefully to ensure that the promise of extended and quality life spans for cancer patients is not lost amid the technical and logistical hurdles.
Car T-cell therapy offers a glimpse of what may soon become a cornerstone in the fight against both blood and solid cancers. Its ability to extend life, improve quality, and potentially redefine treatment protocols resonates with the growing momentum in personalised medicine. Though the challenges are intimidating, every incremental improvement is a step closer to academic, clinical, and practical breakthroughs that might one day make this therapy commonplace in oncology departments around the globe.
As the journey continues, we are reminded that each medical advance—no matter how promising—comes wrapped in a package of tricky parts, tangled issues, and significant efforts from the research community. It is through close collaboration, robust clinical trials, and a steadfast dedication to improving patient outcomes that the future of oncology will be reshaped. The transformative potential of Car T-cell therapy is undeniable, offering hope to many who have long sought answers in a field loaded with tension and uncertainty.
In this new era of cancer treatment, Car T-cell therapy stands as an example of how innovative science can transform lives. While the battle against cancer is far from over, these early results pave the way for an era in which customised, effective, and life-extending therapies become not just a possibility, but a standard of care for patients around the world.
Originally Post From https://www.theguardian.com/science/2025/may/31/immunotherapy-trial-helps-cancer-patients-with-tumours-live-40-longer
Read more about this topic at
Groundbreaking Trial Shows How Immunotherapy …
Immunotherapy Could Replace Surgery, Enabling Patients …